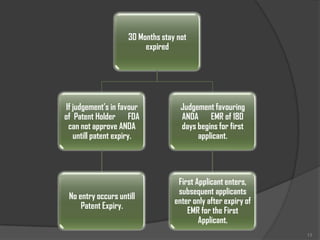
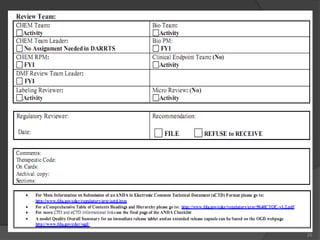
The document details the process and regulations surrounding abbreviated new drug applications (ANDA) for the approval of generic drugs by the FDA, emphasizing their equivalence to innovator drugs in terms of active ingredients, safety, and efficacy, but with reduced costs. It outlines the Hatch-Waxman Act, which streamlined the approval process and facilitated the introduction of generic drugs in the U.S. market by reducing the need for extensive clinical trials. Additionally, it discusses the bioequivalence of generic drugs and the structural requirements for submission, including content and modules for electronic submissions.