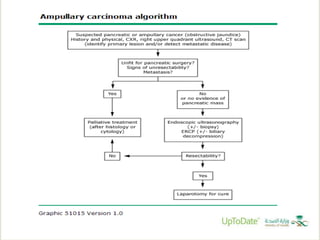
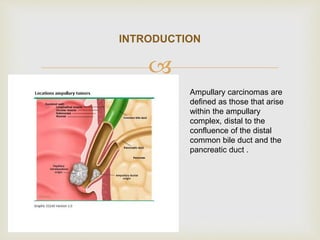
This document discusses ampullary carcinomas, including their epidemiology, clinical manifestations, diagnosis, staging, treatment, and prognosis. It provides details on: the average age of diagnosis being 60-70 years old; the most common histologic subtype being intestinal (47%); obstructive jaundice being the most common presenting symptom (80%); diagnostic tests including ERCP, CT, and tumor markers; the TNM staging system; pancreaticoduodenectomy being the standard treatment for localized disease; and adjuvant therapy options including chemotherapy and chemoradiotherapy for stage IB or higher cancers.


![
Subdividing adenocarcinomas of the ampulla of Vater according to
histologic subtype and immunohistochemical staining pattern into
distinct subsets with differing biologic behavior has prognostic
importance.
In a retrospective study of 208 patients treated for ampullary
adenocarcinoma in Sydney, Australia,
those with a histomolecular pancreaticobiliary phenotype (CDX-
negative, MUC1 positive) had a significantly worse outcome
than did those with an intestinal phenotype (CDX-positive, MUC1-
negative), with median survival of 16 versus 116 months [22].
EPIDEMIOLOGY AND BIOLOGIC BEHAVIOR](https://image.slidesharecdn.com/ampullarycarcinoma-140827105523-phpapp02/85/Ampullary-carcinoma-9-320.jpg)
![ Most common presenting symptom of ampullary carcinoma is obstructive jaundice
(80 percent)
Additional symptoms may include diarrhea due to fat malabsorption (steatorrhea),
mild weight loss, and fatigue.
Up to one-third of patients have chronic, frequently occult gastrointestinal blood loss
with an associated microcytic anemia or heme-positive stools.
occasionally present with frank bleeding due to sloughing of the tumor, a condition
exacerbated by the use of antiplatelet agents such as aspirin and clopidogrel.
In one report, nonspecific symptoms include abdominal pain (45 percent), fever (45
percent), mild nausea, and dyspepsia [25].
Large lesions may produce gastric outlet obstruction associated with severe nausea
and vomiting
CLINICAL MANIFESTATIONS](https://image.slidesharecdn.com/ampullarycarcinoma-140827105523-phpapp02/85/Ampullary-carcinoma-11-320.jpg)




![
Endoscopic snare resection (papillectomy) is an effective means of treating
ampullary adenomas.
Endoscopic papillectomy has been attempted in early stage (Tis, T1) well
differentiated ampullary cancers without angiolymphatic invasion
Endoscopic debulking has been used mainly preoperatively to permit stent
insertion and decompression of the biliary tree.
Laser ablation offers the potential for control of local tumor growth in
patients who are unfit for more aggressive therapy. In one such series of
12 patients with ampullary cancer, duodenal obstruction was relieved in
one, and the longest survival was 36 months (median 21 months) [45].
●Compared with laser ablation, photodynamic therapy (PDT) eradicates
local tumor with less surrounding tissue destruction. PDT uses a
photosensitizing drug (a hematoporphyrin derivative, Photofrin®), which is
disproportionately retained by malignant tissue after intravenous
administration.](https://image.slidesharecdn.com/ampullarycarcinoma-140827105523-phpapp02/85/Ampullary-carcinoma-26-320.jpg)
![ Pancreaticoduodenectomy rather than local resection for most patients with invasive
ampullary carcinomas (Grade 1B).
Local ampullary excision rather than pancreaticoduodenectomy for patients with
noninvasive ampullary tumors.
Ampullectomy is also a reasonable approach for poor surgical candidates who have
a well-differentiated T1 tumor that is less than 6 mm in size (based upon endoscopic
ultrasound [EUS]).
However, a more aggressive surgical approach is preferred for patients who are
candidates for pancreaticoduodenectomy because of better outcomes.
Nonsurgical treatment modalities (ie, endoscopic snare resection, laser ablation,
photodynamic therapy) provide palliative rather than curative benefit for patients with
ampullary carcinoma.
These methods should be restricted to patients who are not operative candidates
and those who refuse surgery.
Primary treatment](https://image.slidesharecdn.com/ampullarycarcinoma-140827105523-phpapp02/85/Ampullary-carcinoma-27-320.jpg)