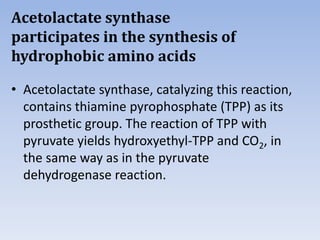
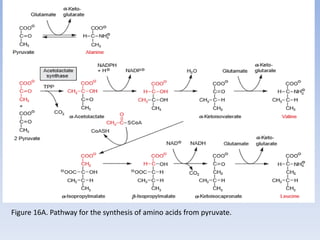
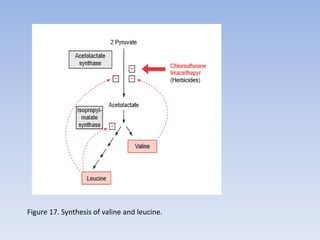
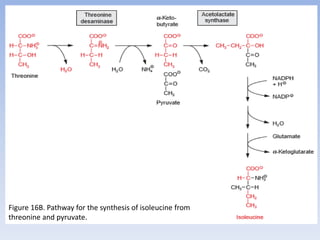
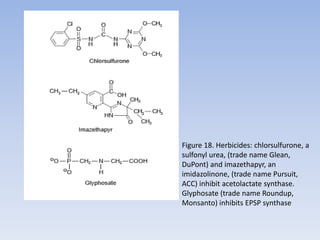
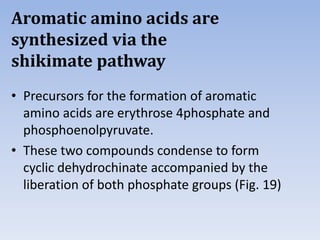
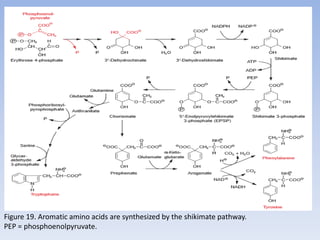
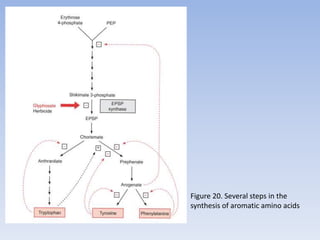
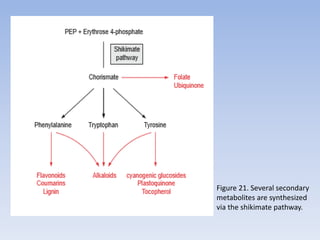
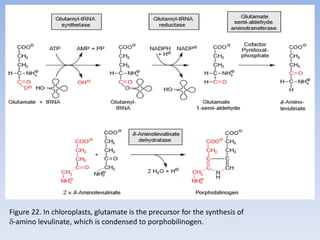
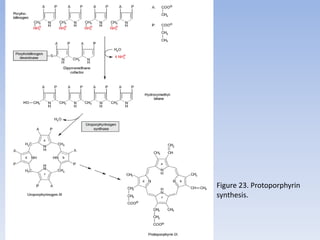
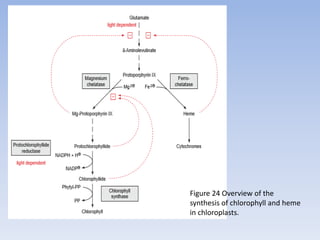
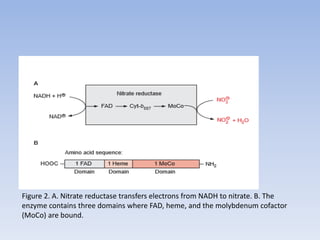
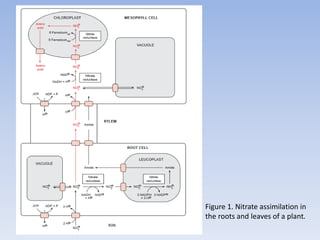
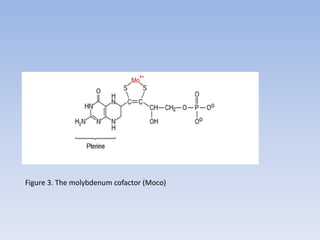
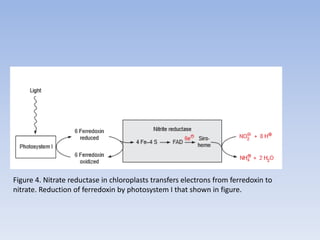
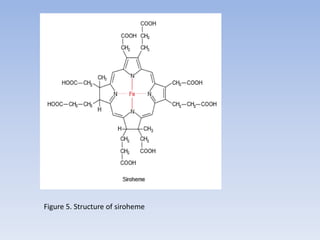
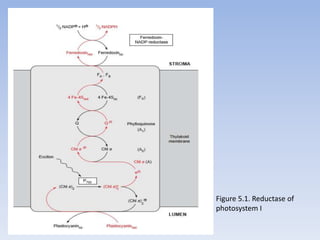
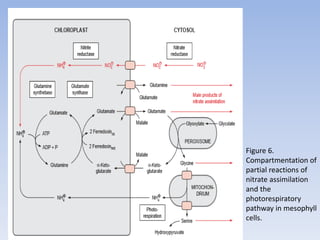
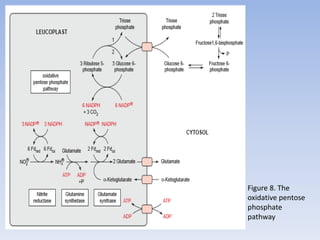
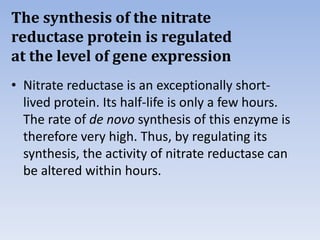
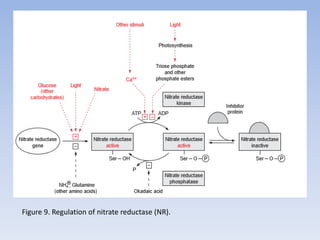
Nitrate assimilation is essential for synthesizing organic nitrogen compounds like amino acids from inorganic nitrogen, primarily involving plants, fungi, and certain bacteria. This process occurs notably in roots and leaves where nitrate is absorbed and converted into ammonia for amino acid synthesis. Approximately 99% of organic nitrogen in the biosphere originates from nitrate assimilation, illustrating a continuous cycle of nitrogen between soil and plants.















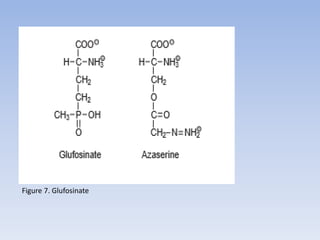










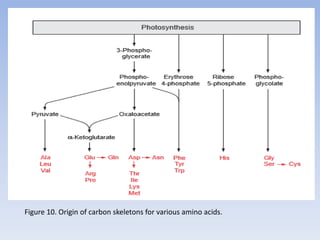
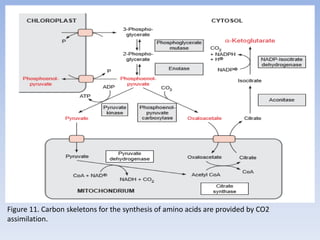


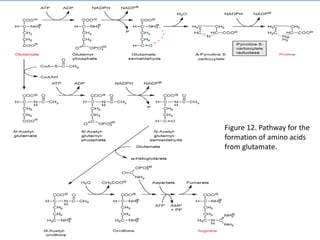

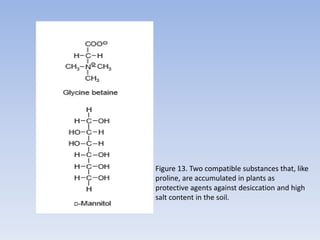
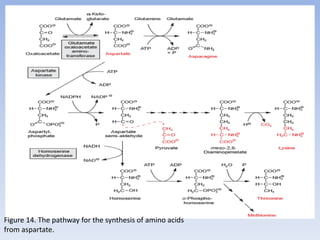
![Figure 15. Feedback inhibition by end products regulates the entrance enzyme for
the synthesis of amino acids from aspartate according to demand. [-] indicates
inhibition. Aspartate kinase exists in two isoforms](https://image.slidesharecdn.com/cs701nitrateassimilationarnolddamaso-160108012300/85/CS_701_Nitrate-Assimilation-by-arnold_damaso-45-320.jpg)