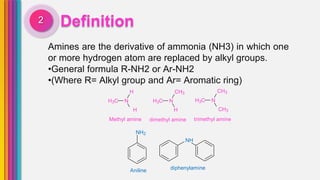
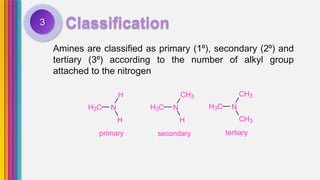
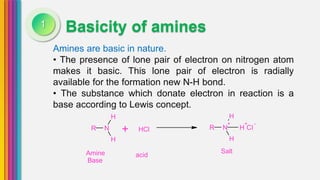
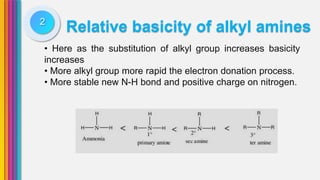
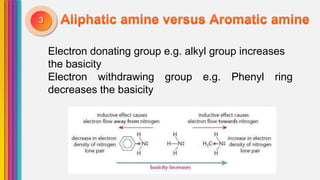
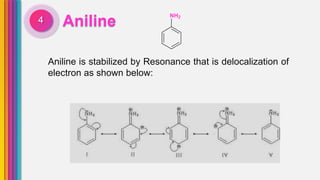
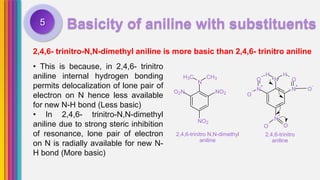
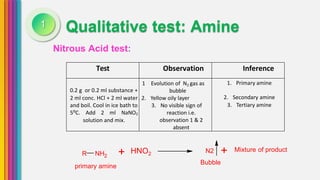
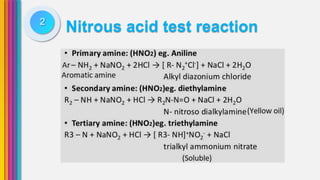
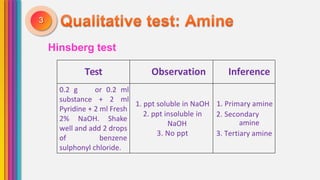
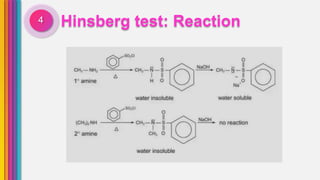
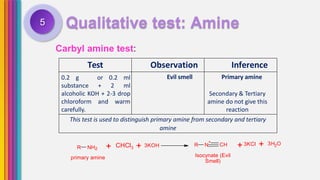
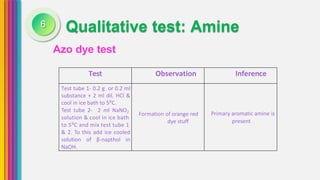
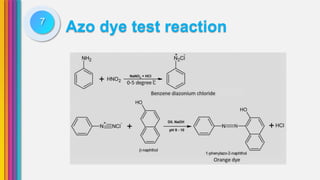
The document provides an overview of amines, describing their classification as primary, secondary, and tertiary based on the number of alkyl groups attached to nitrogen. It discusses the basicity of amines, highlighting factors that influence it, as well as qualitative tests used to identify different types of amines. Additionally, it outlines the structures and various uses of specific amines such as ethylenediamine and amphetamine.