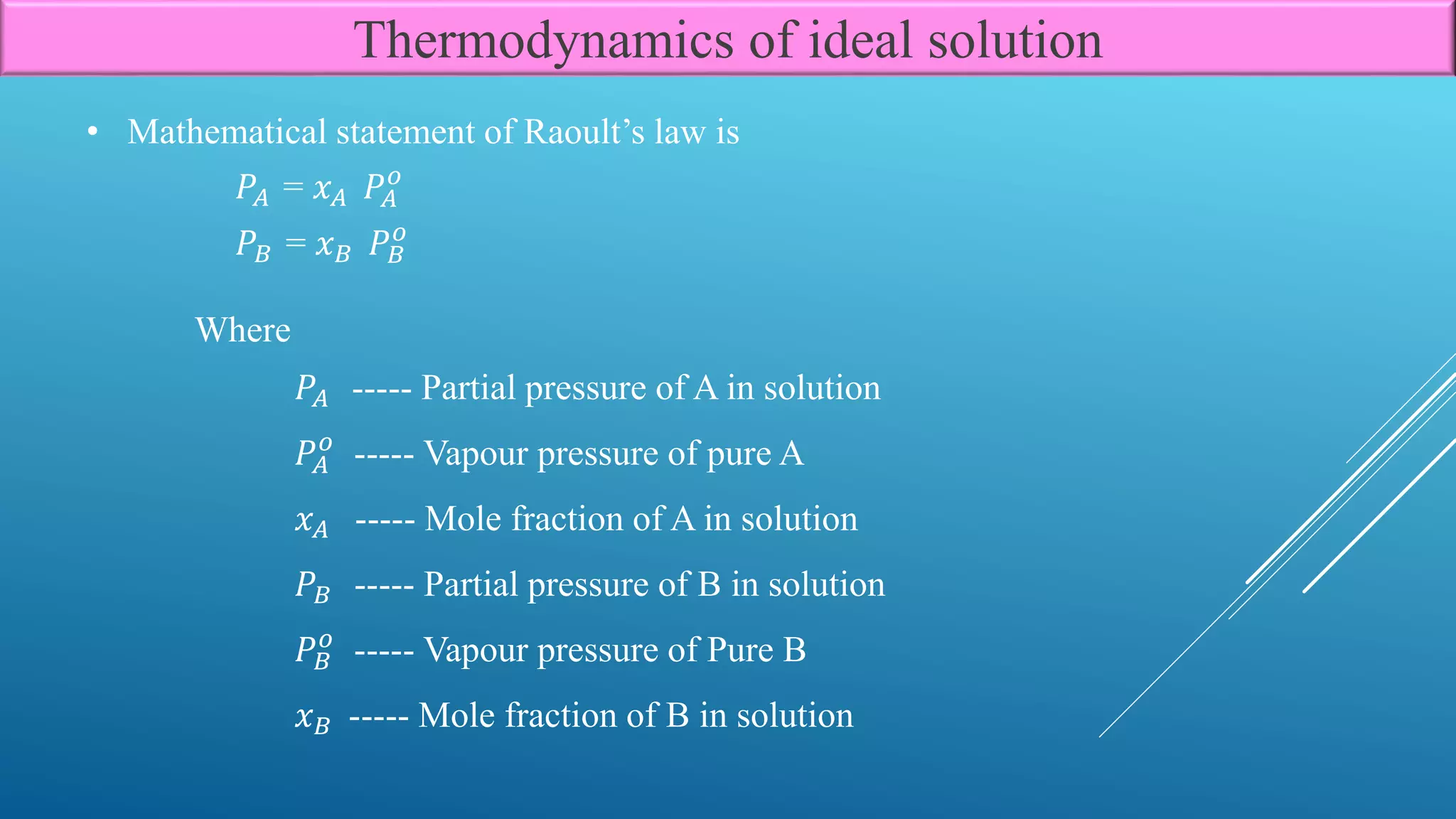
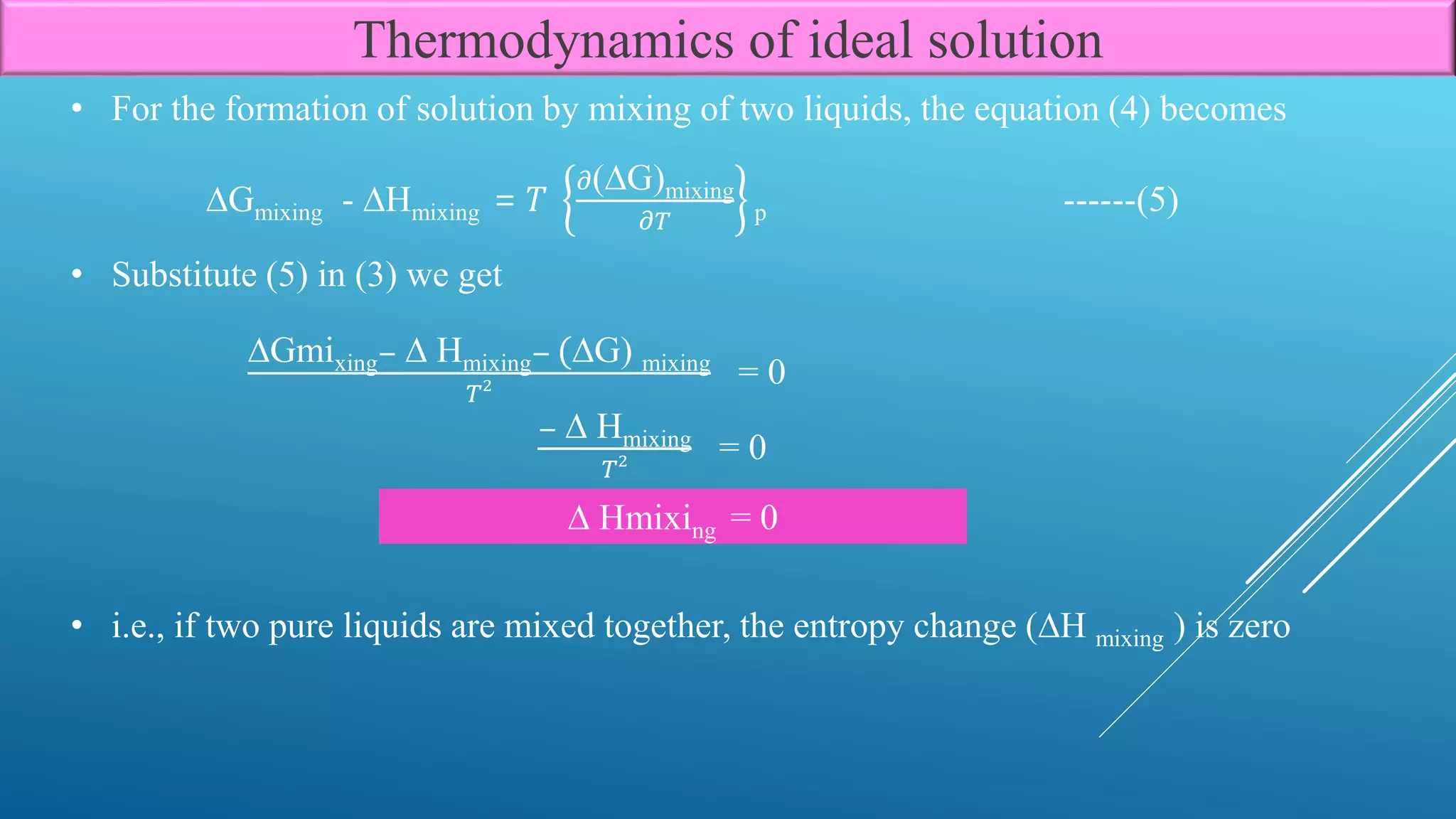
The document discusses the thermodynamics of ideal solutions, focusing on the definitions, mathematical formulations, and laws governing the behavior of solutions. Key concepts include mole fractions, Raoult’s law, and the change in free energy, enthalpy, and entropy during mixing processes. The implications of these principles for various types of solutions, including solid in liquid, liquid in liquid, and gas in liquid, are also examined.