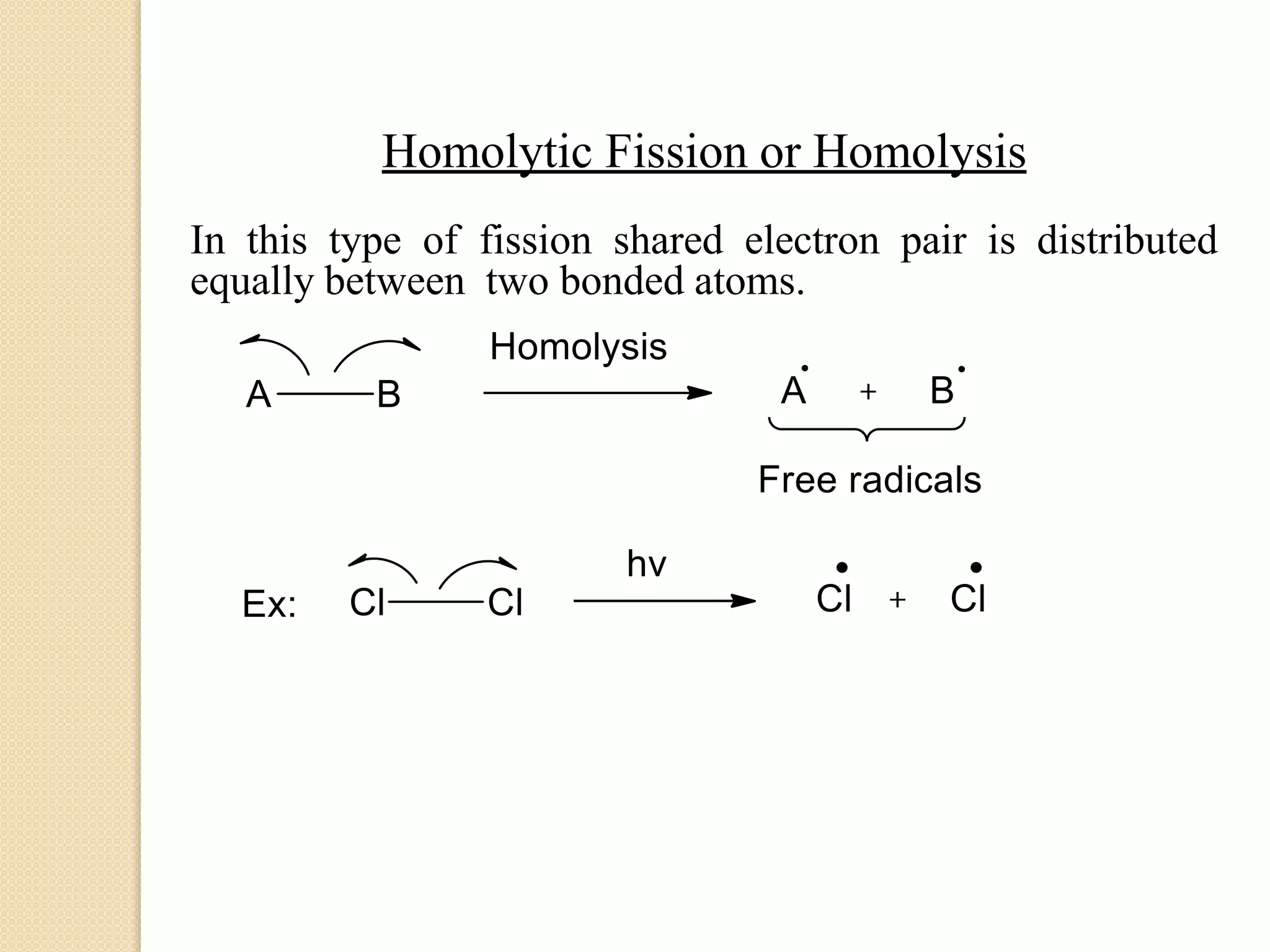
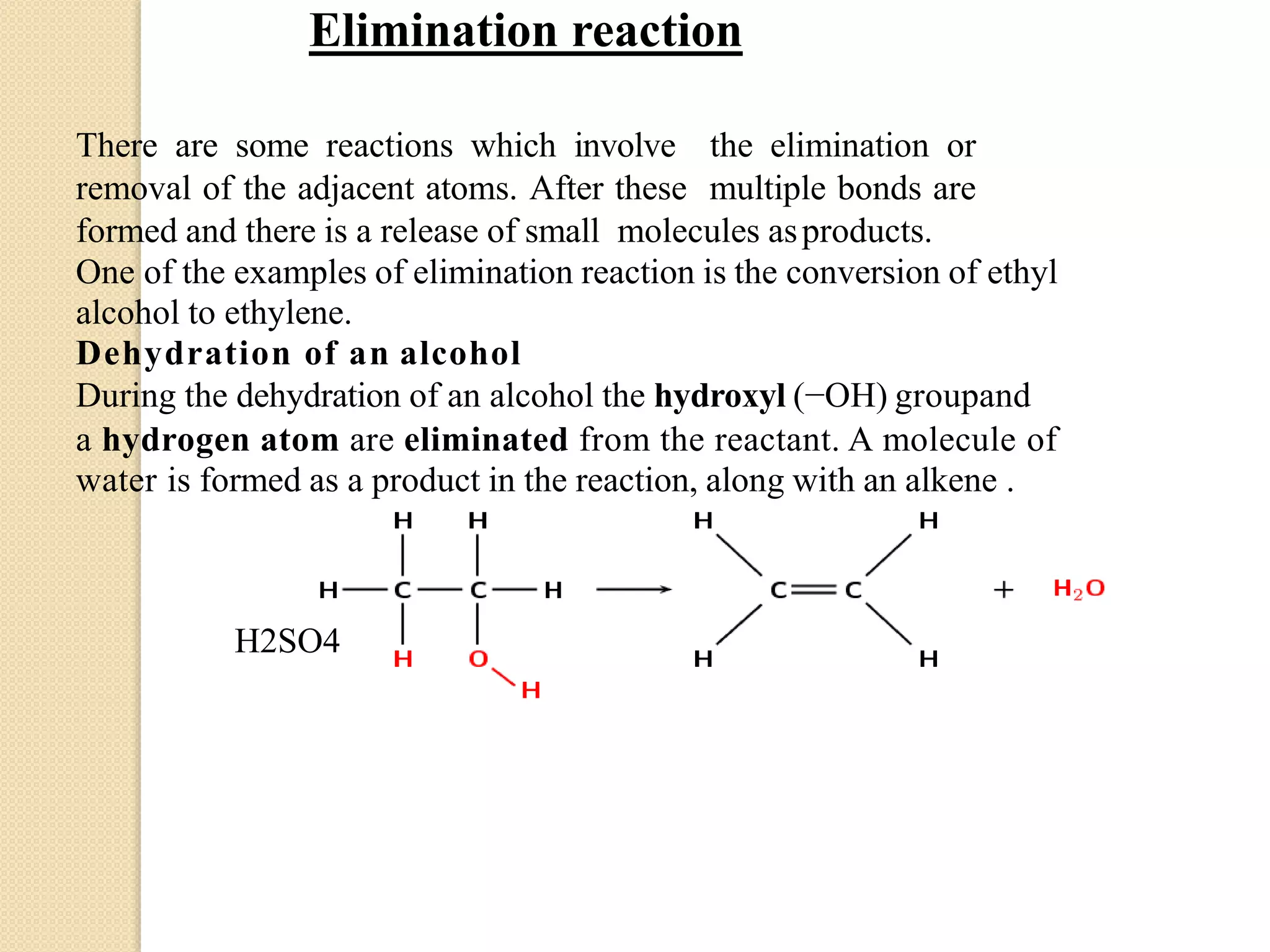
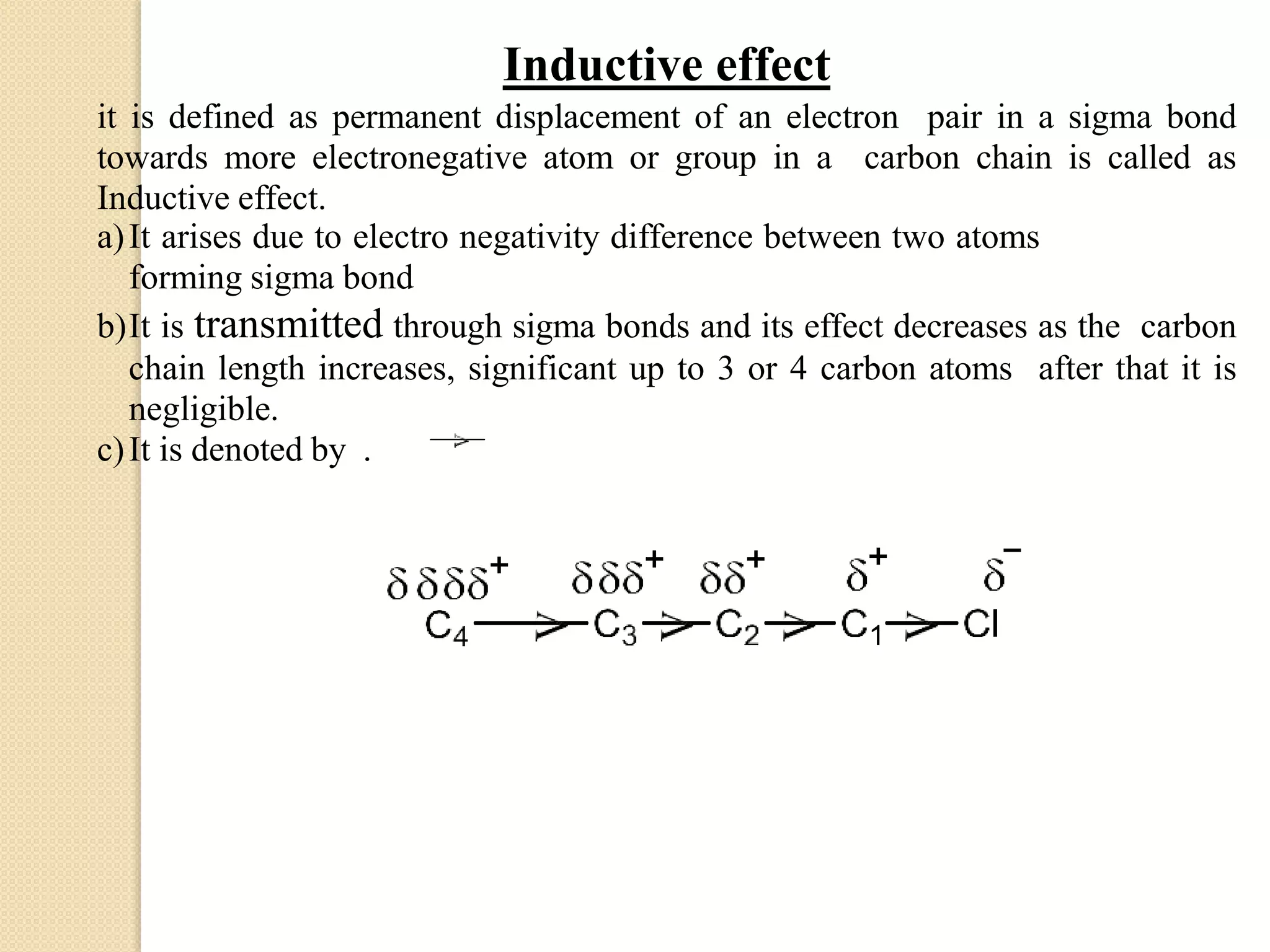
This document discusses structural theory in organic chemistry. It covers topics like bond fission and its types (homolysis and heterolysis), organic reagents (electrophiles, nucleophiles, free radicals), types of organic reactions (substitution, elimination, addition, rearrangement), inductive effect and its applications, and mesomeric effect and its applications. Examples are provided to illustrate key concepts. Essay and short answer questions are also included at the end to test understanding of topics covered.