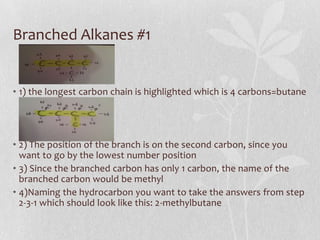
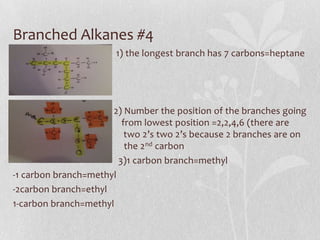
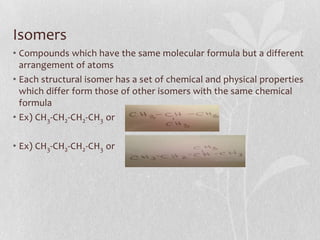
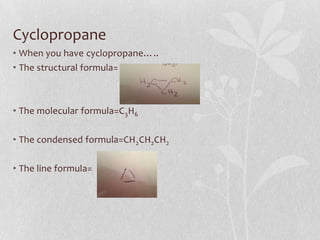
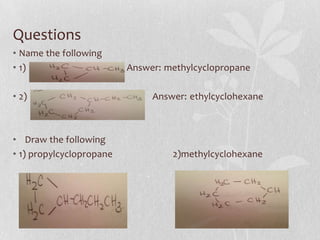
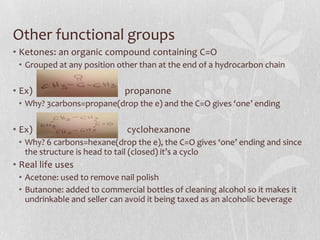
The document discusses naming conventions and structures for organic chemistry compounds including alkanes, haloalkanes, alkenes, and cycloalkanes. It provides prefixes and suffixes used to name compounds based on the number of carbons, functional groups present, and branching. Examples are given for drawing structures and naming compounds with 1-4 carbons as well as branched, cyclic, and halo-substituted variants. Common uses of some compounds are also mentioned.