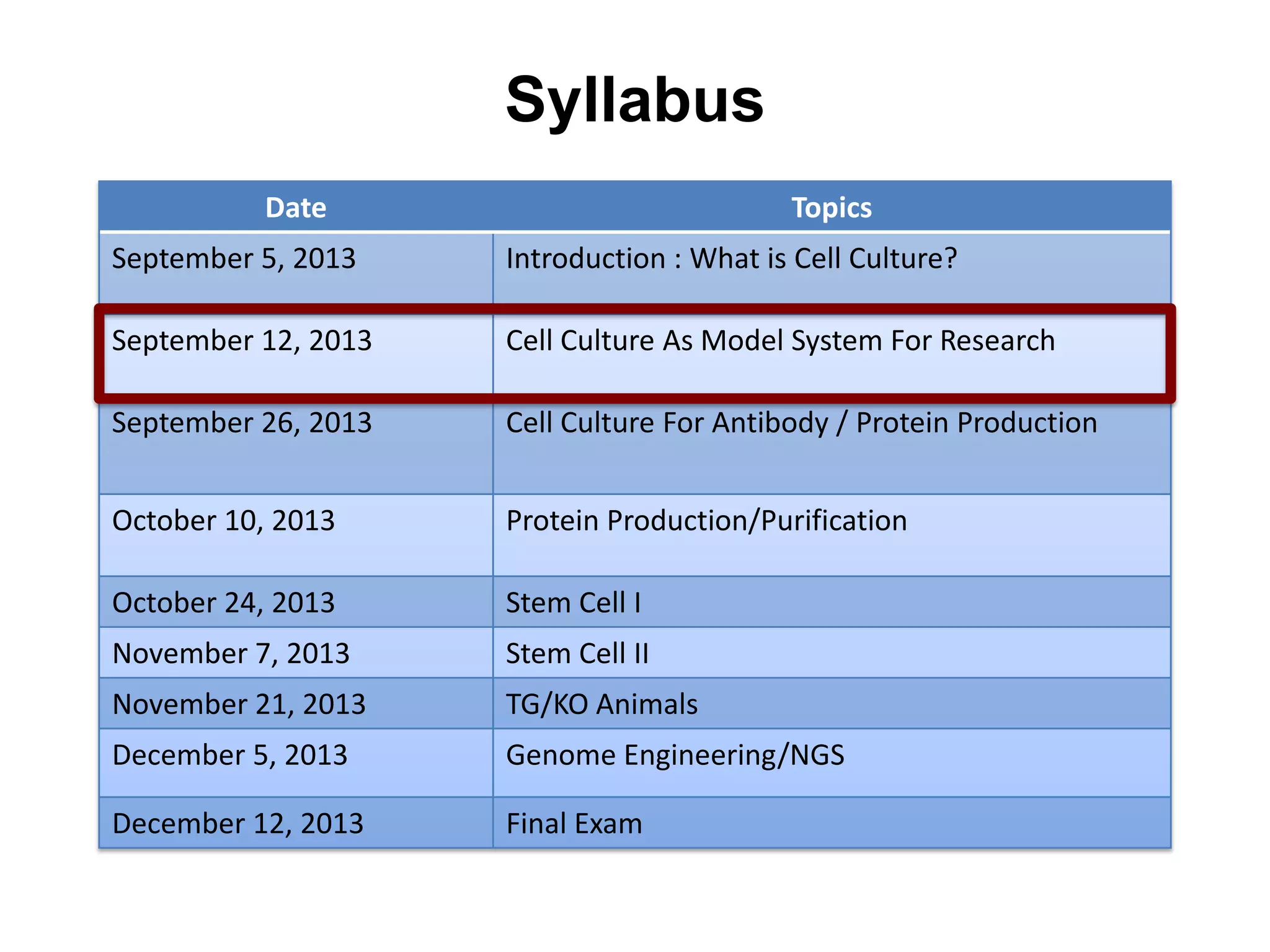
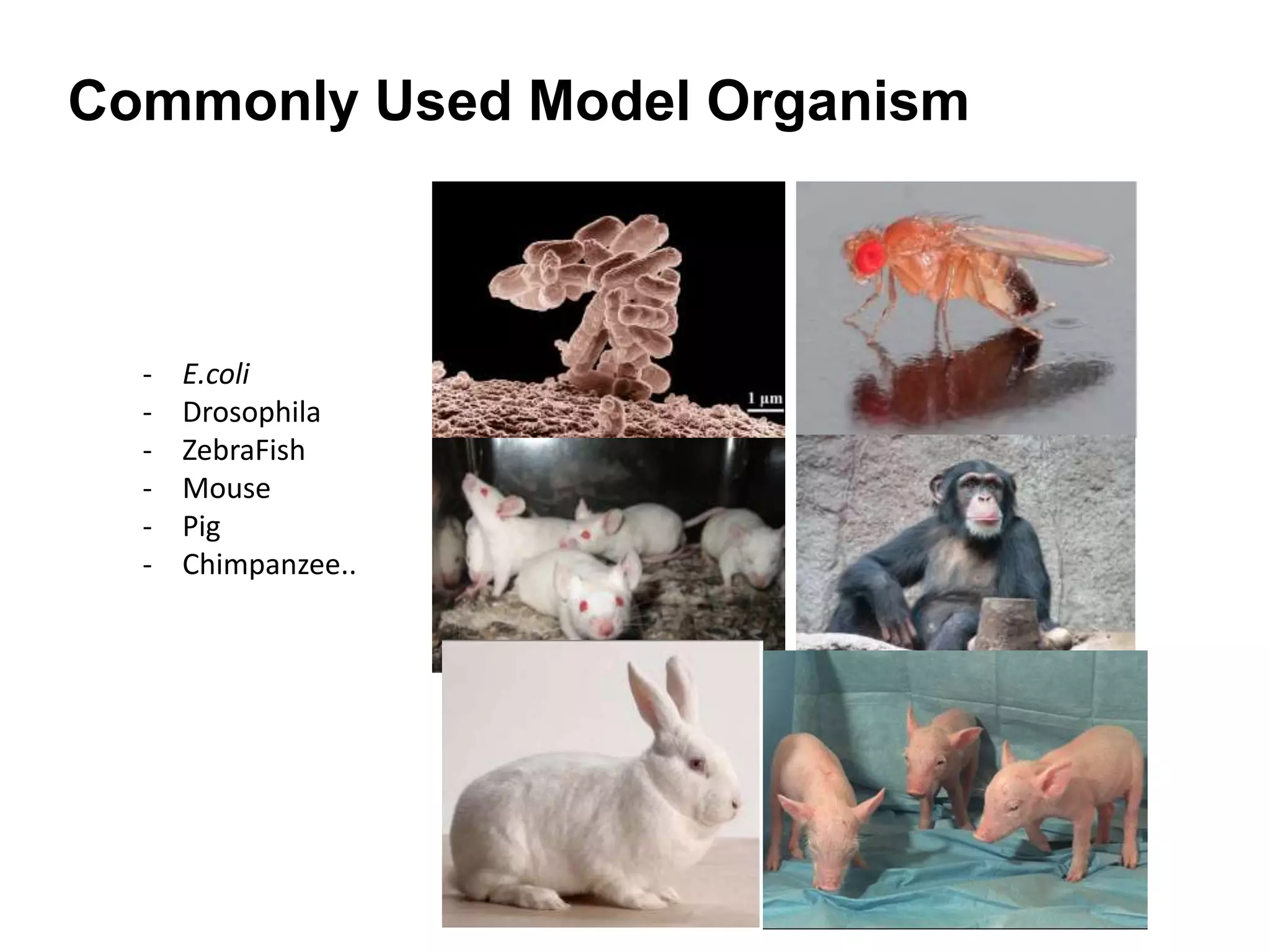
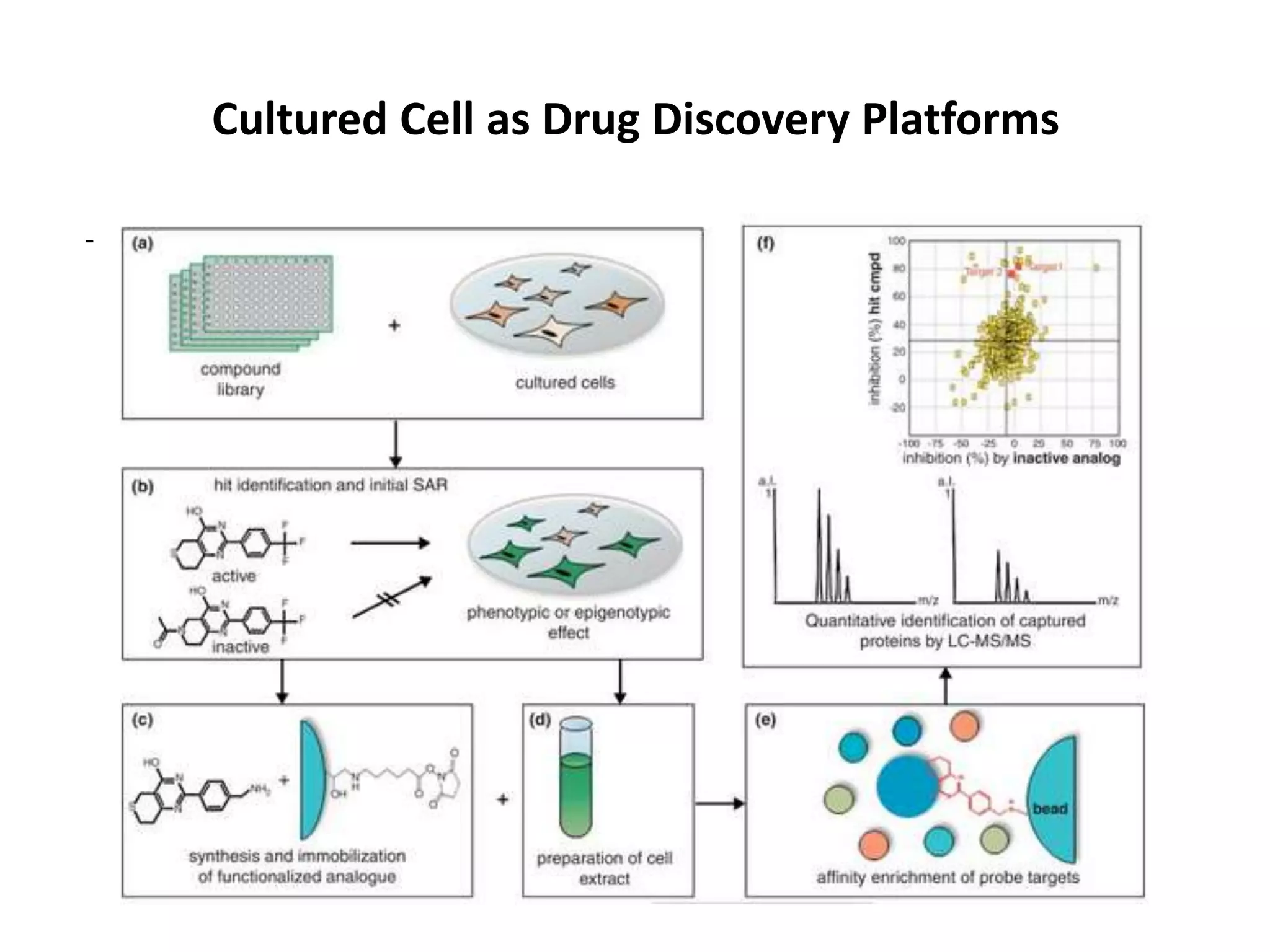
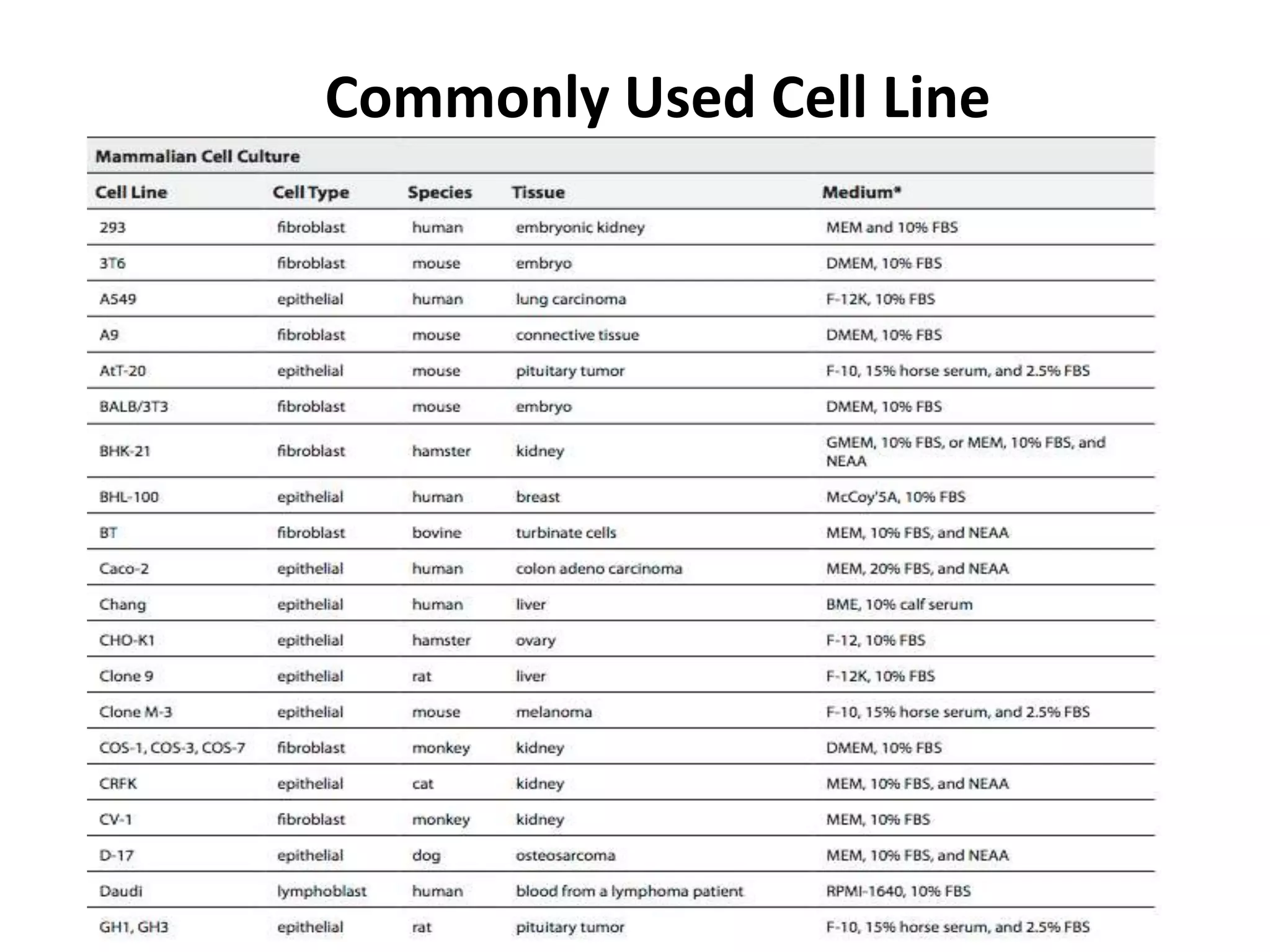
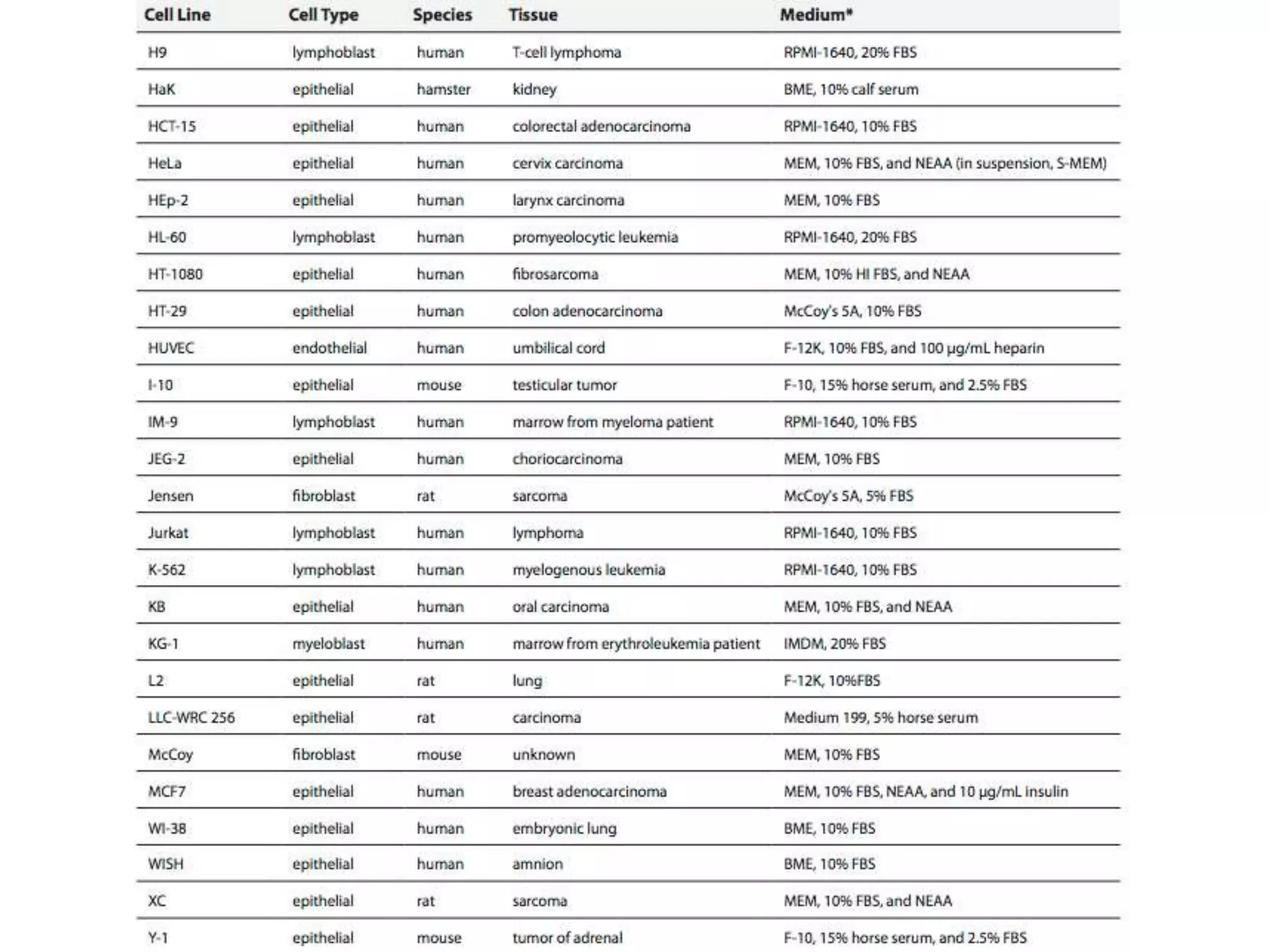
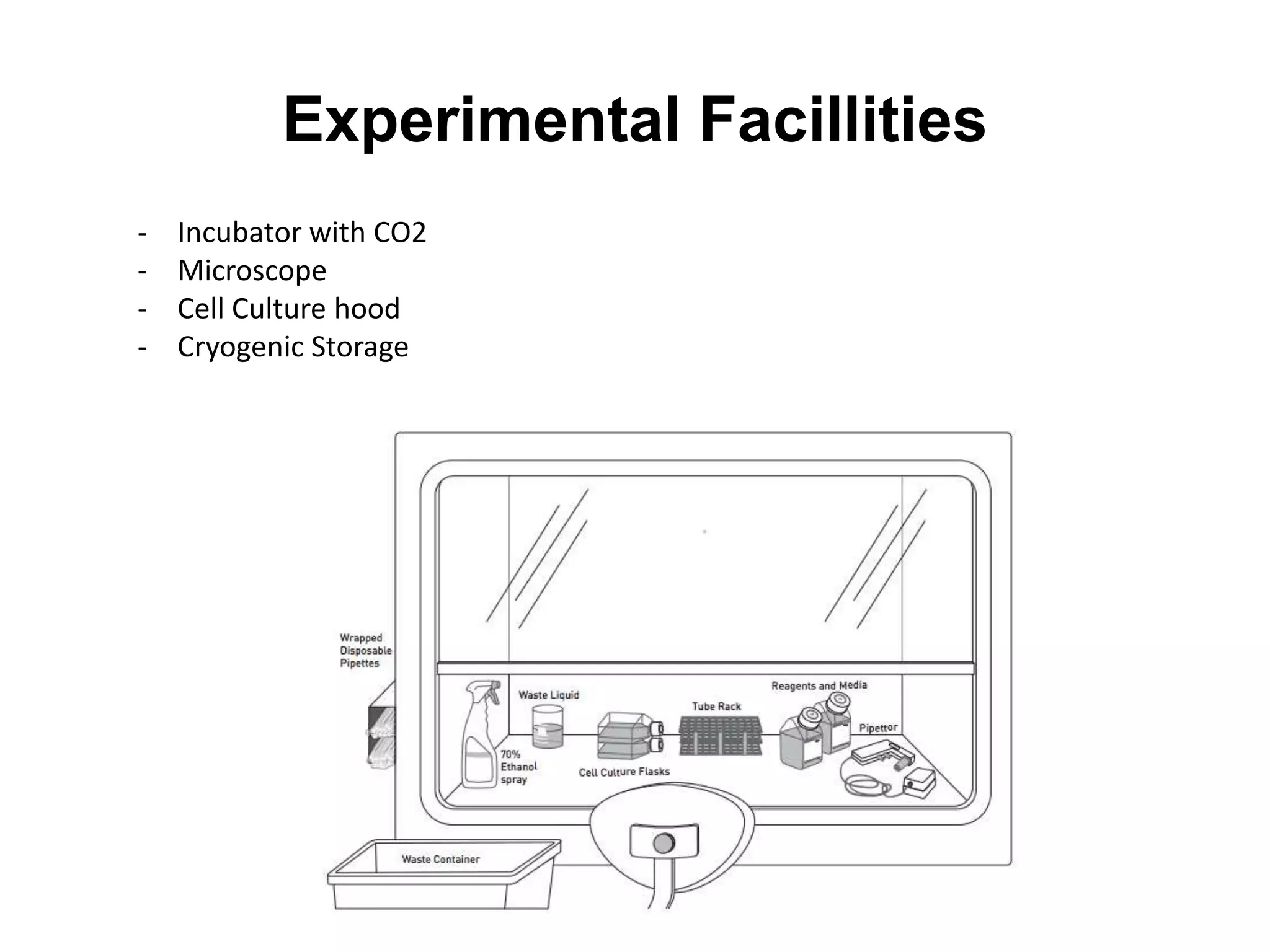
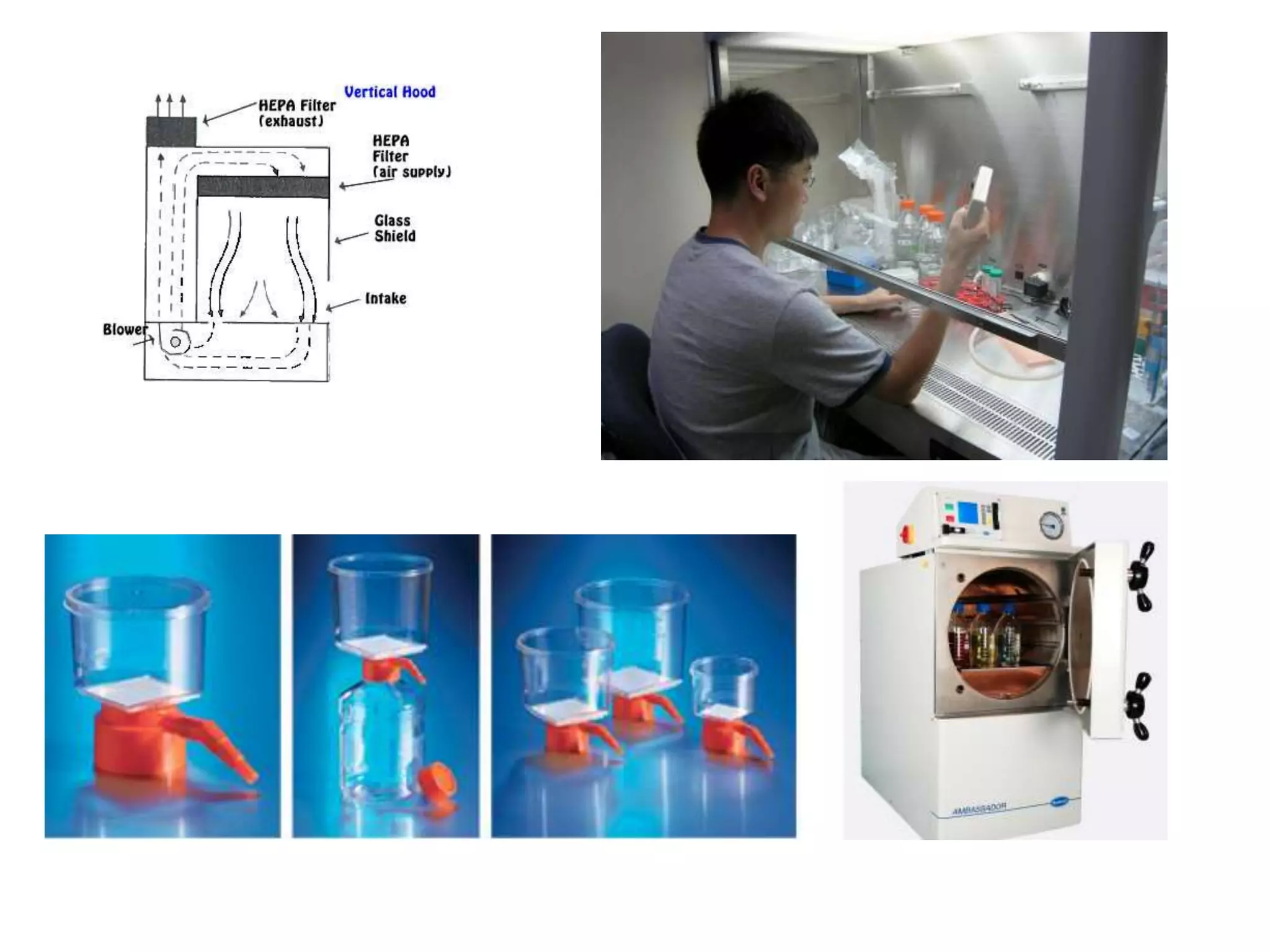
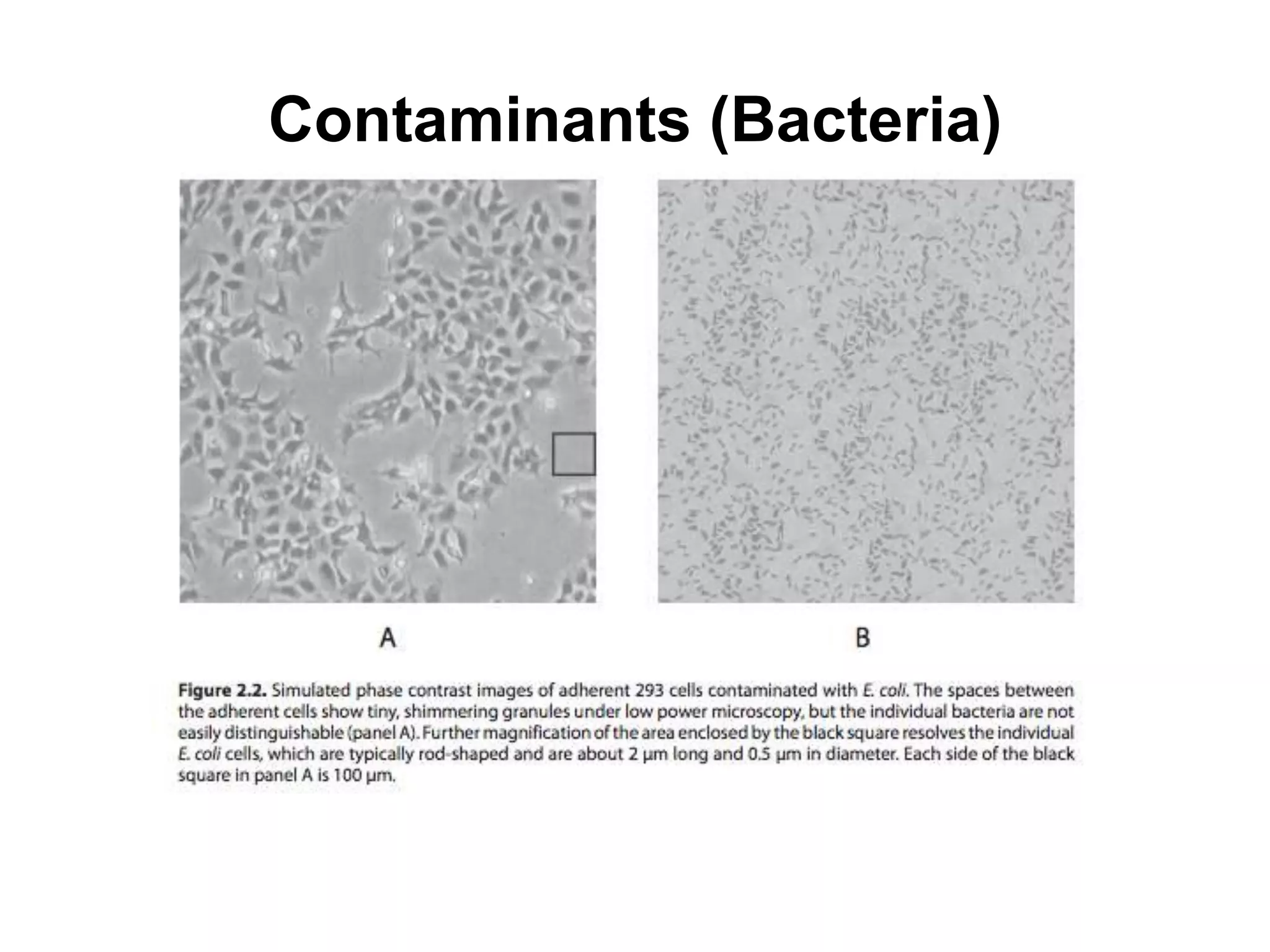
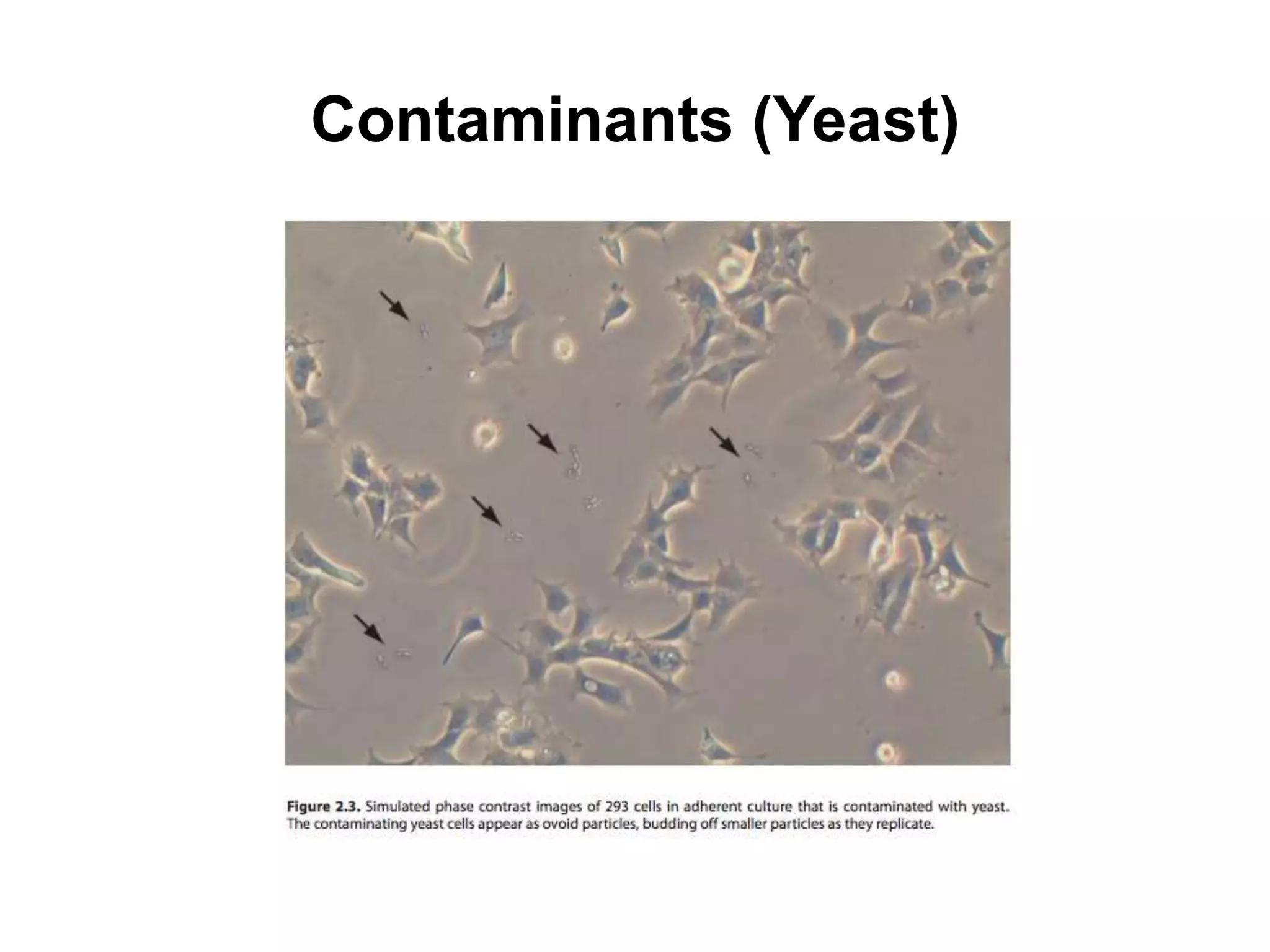
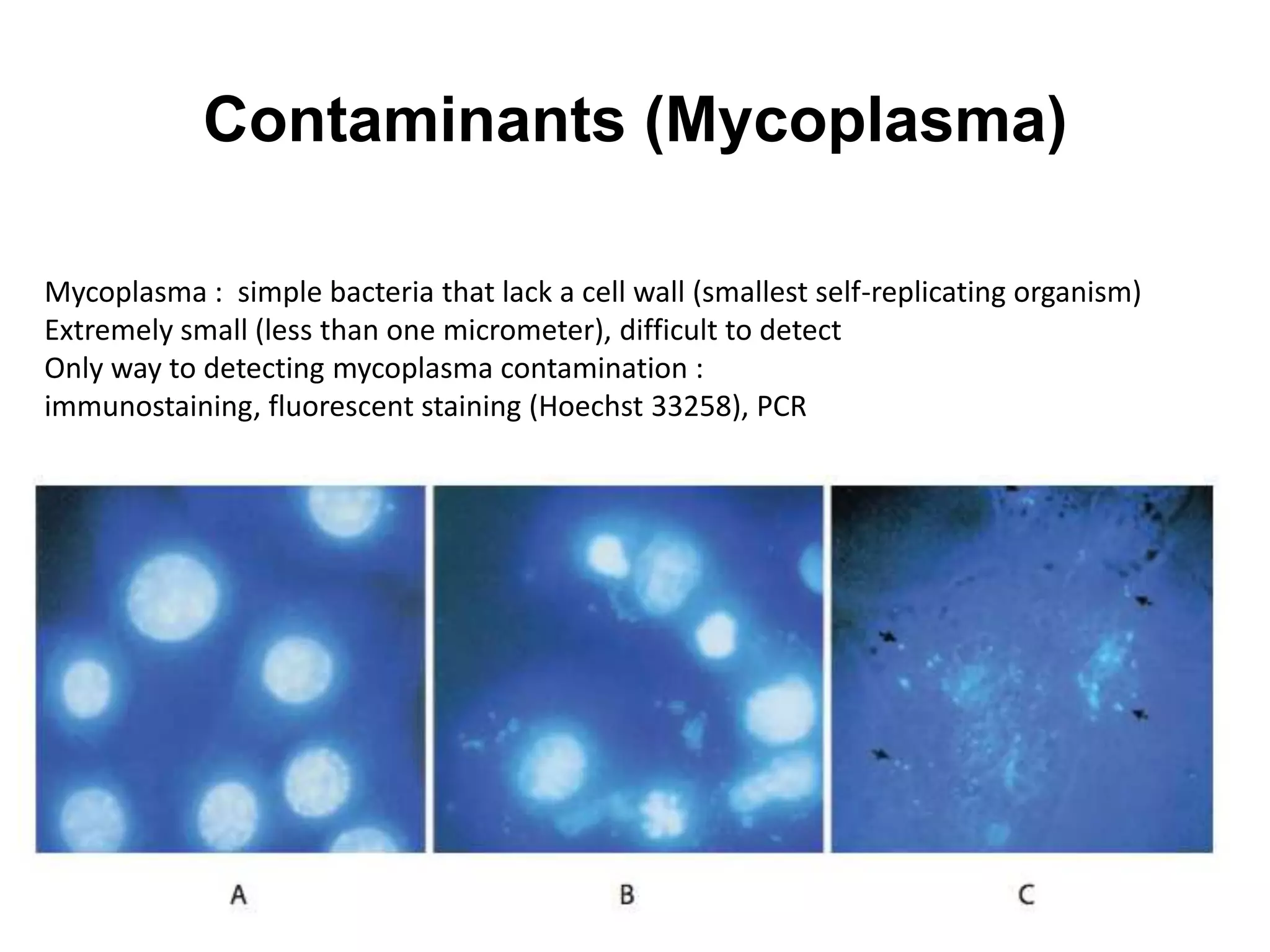
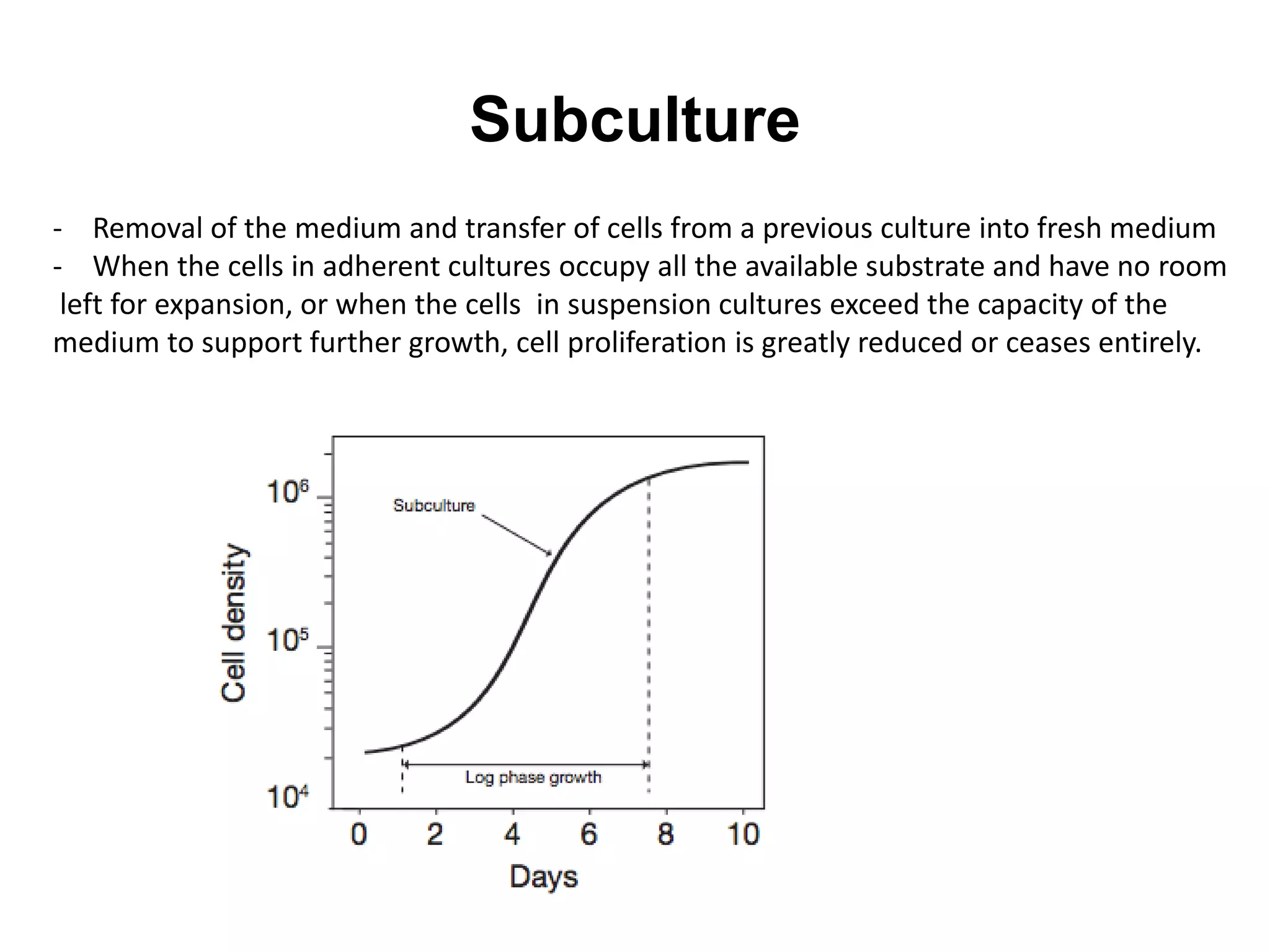
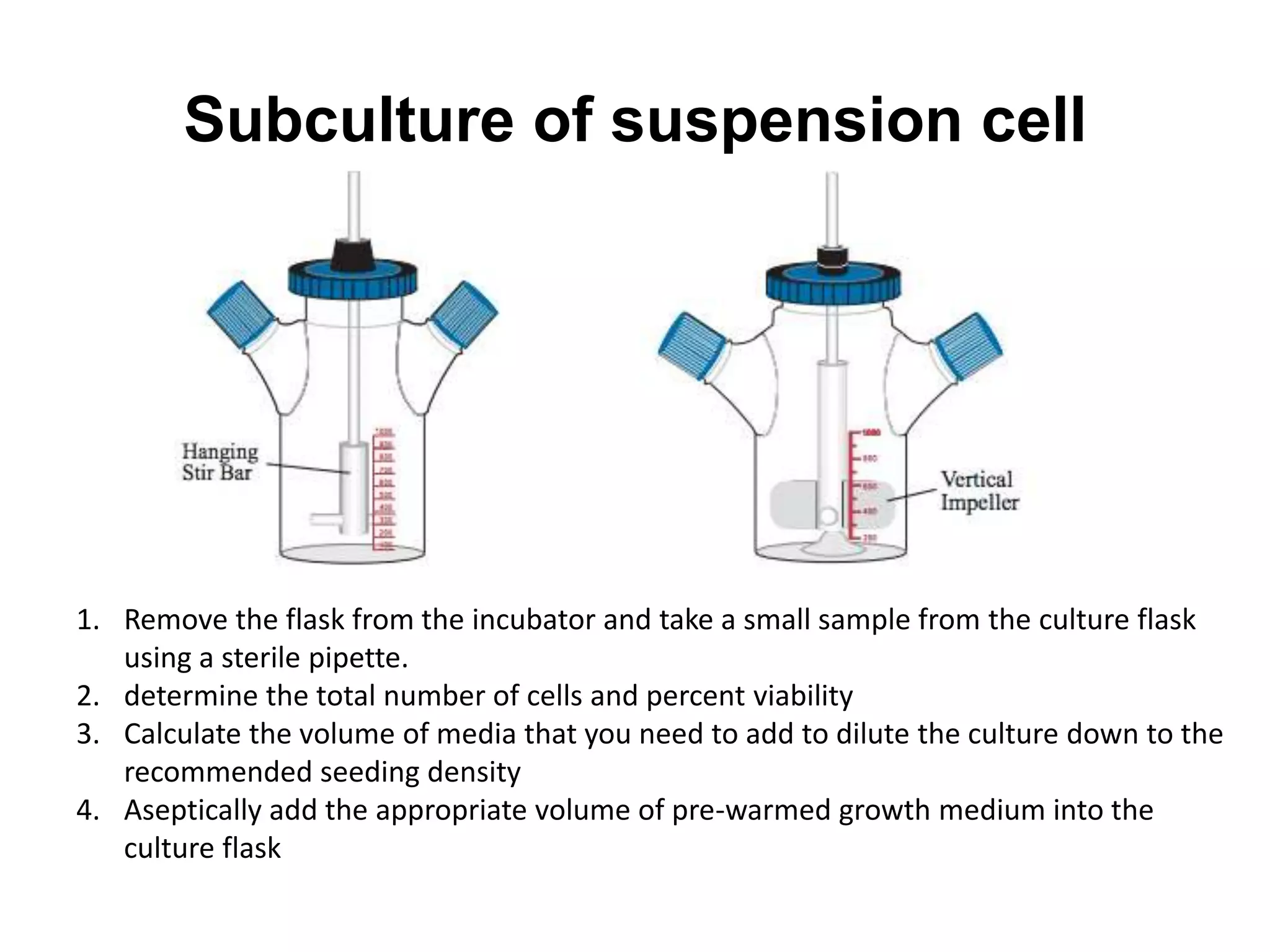
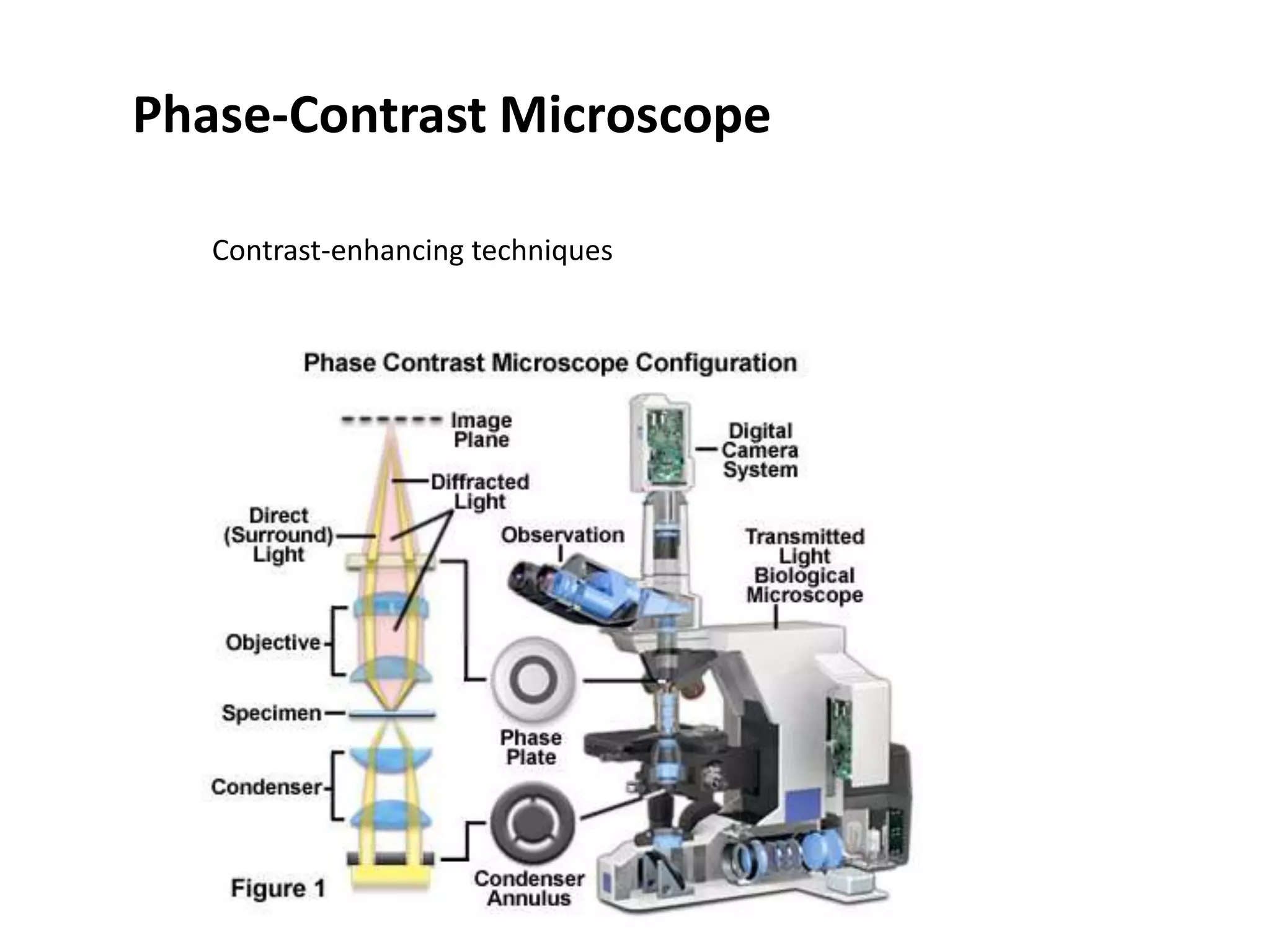
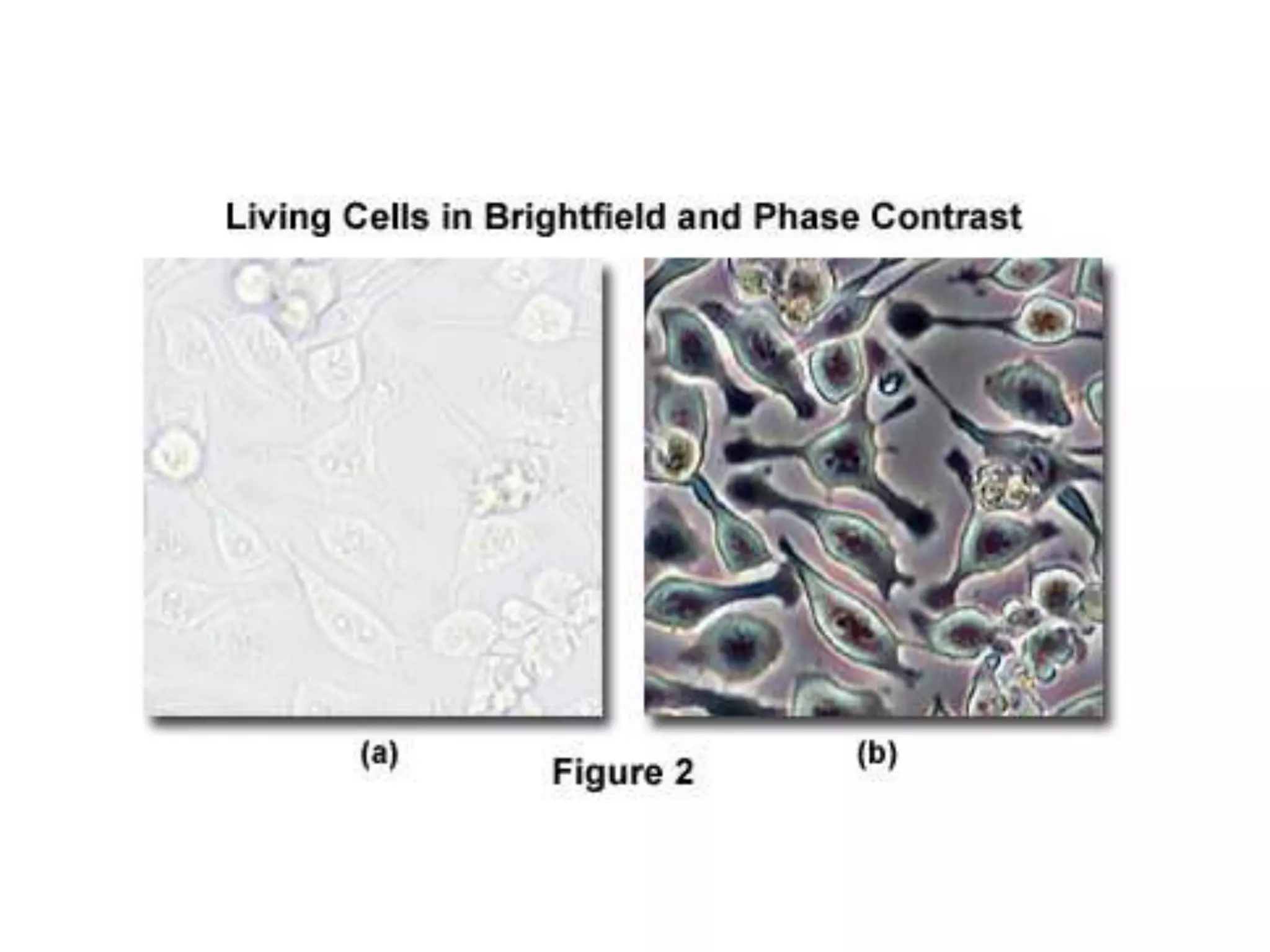
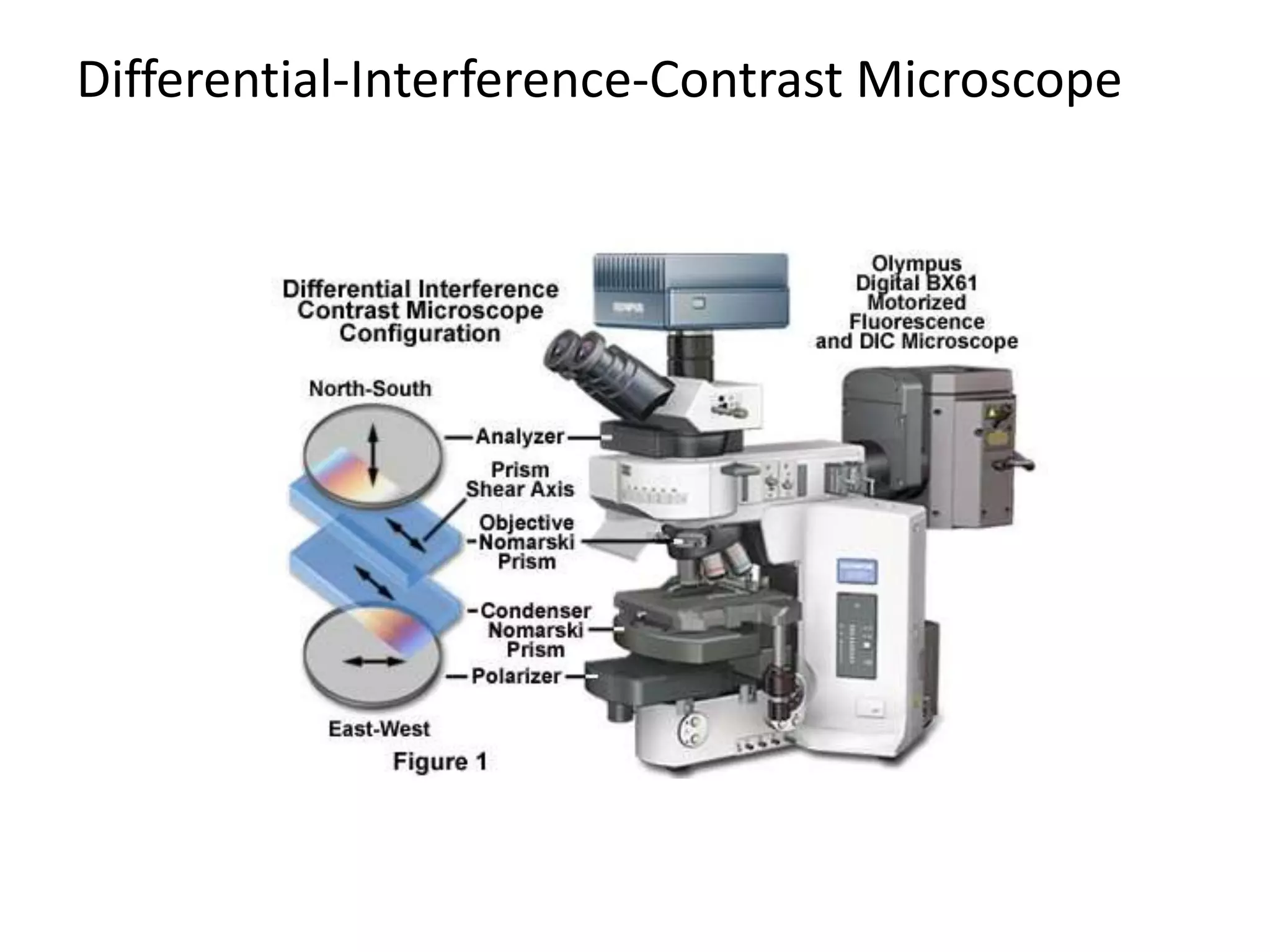
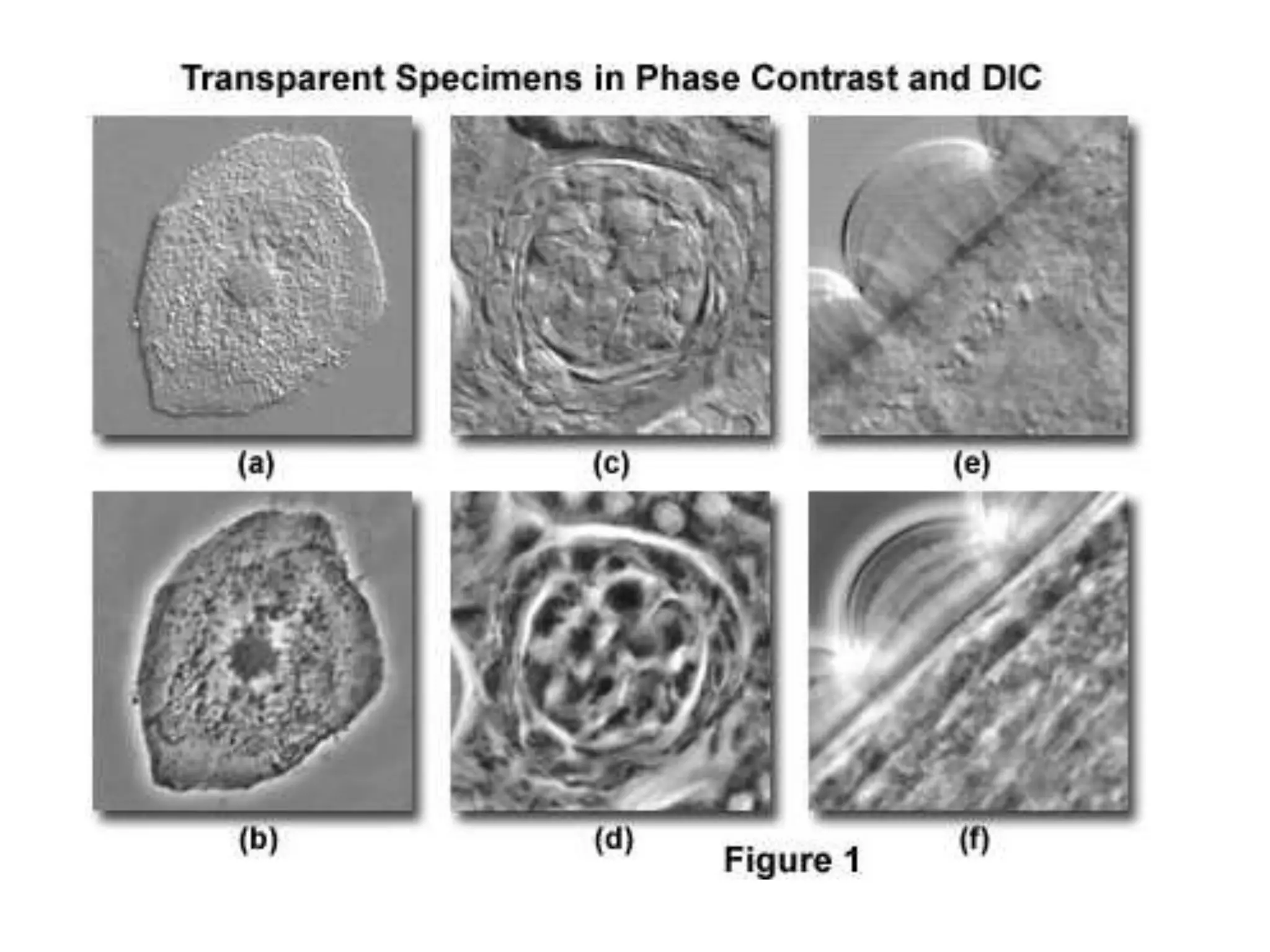
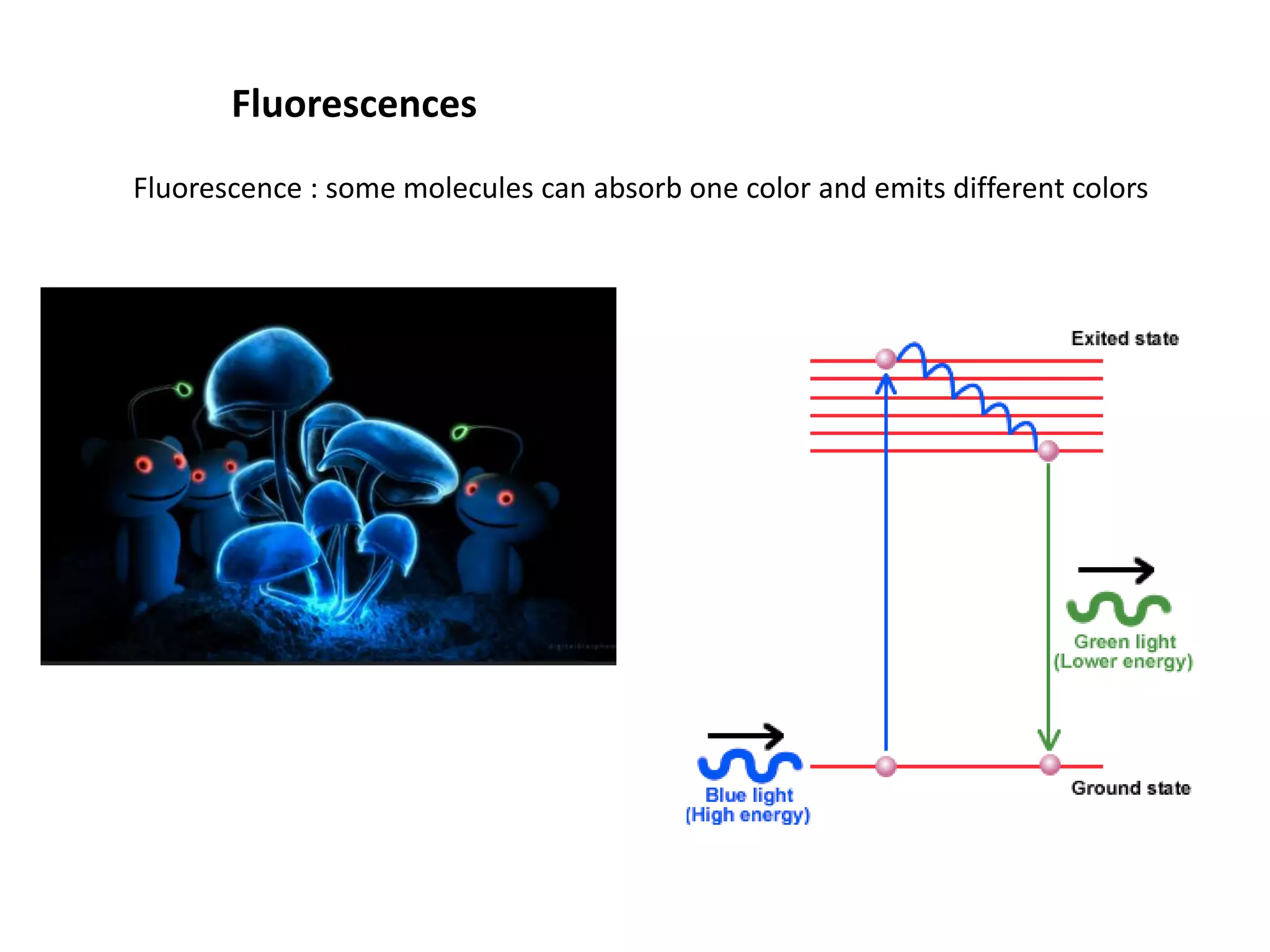
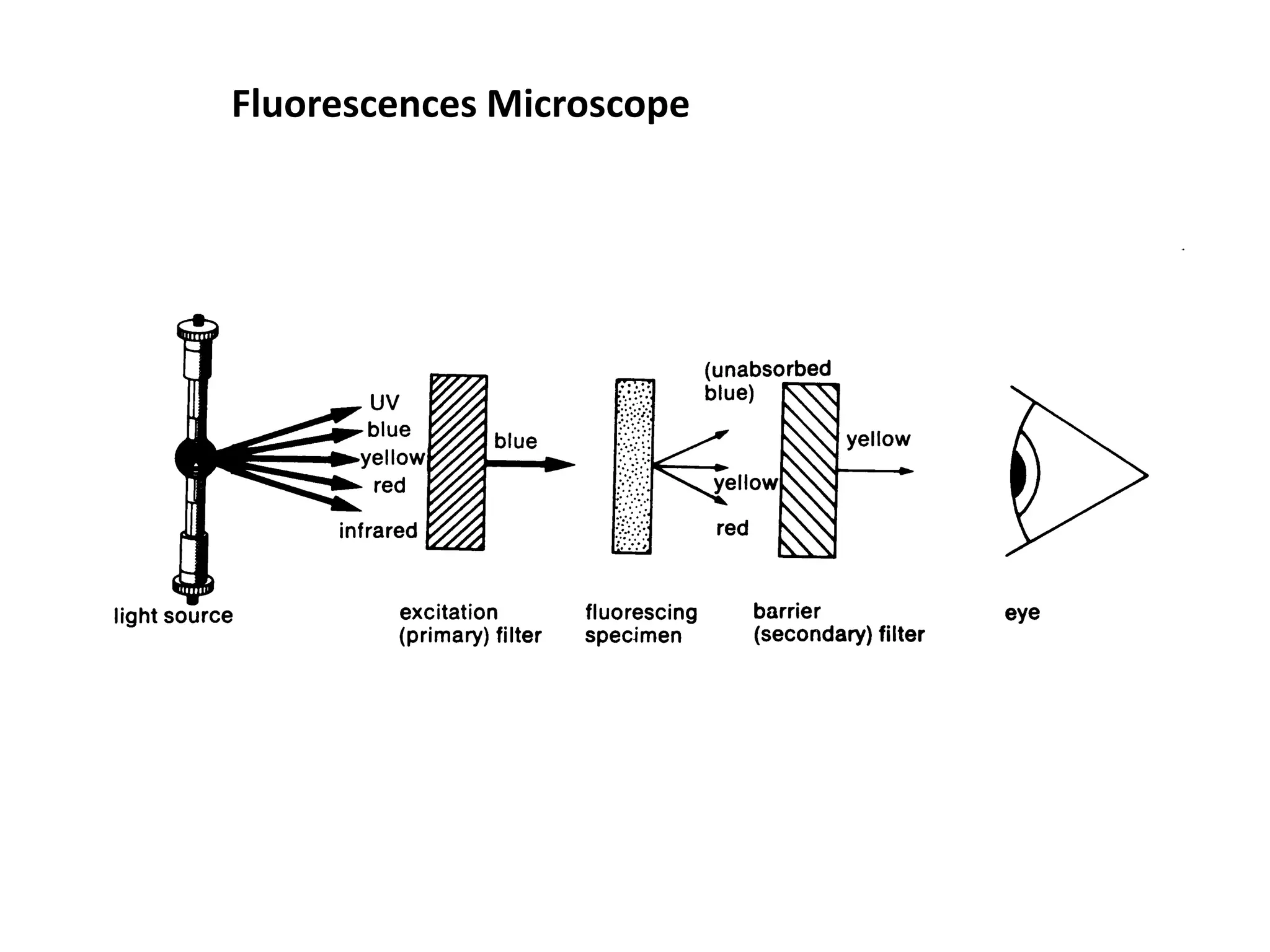
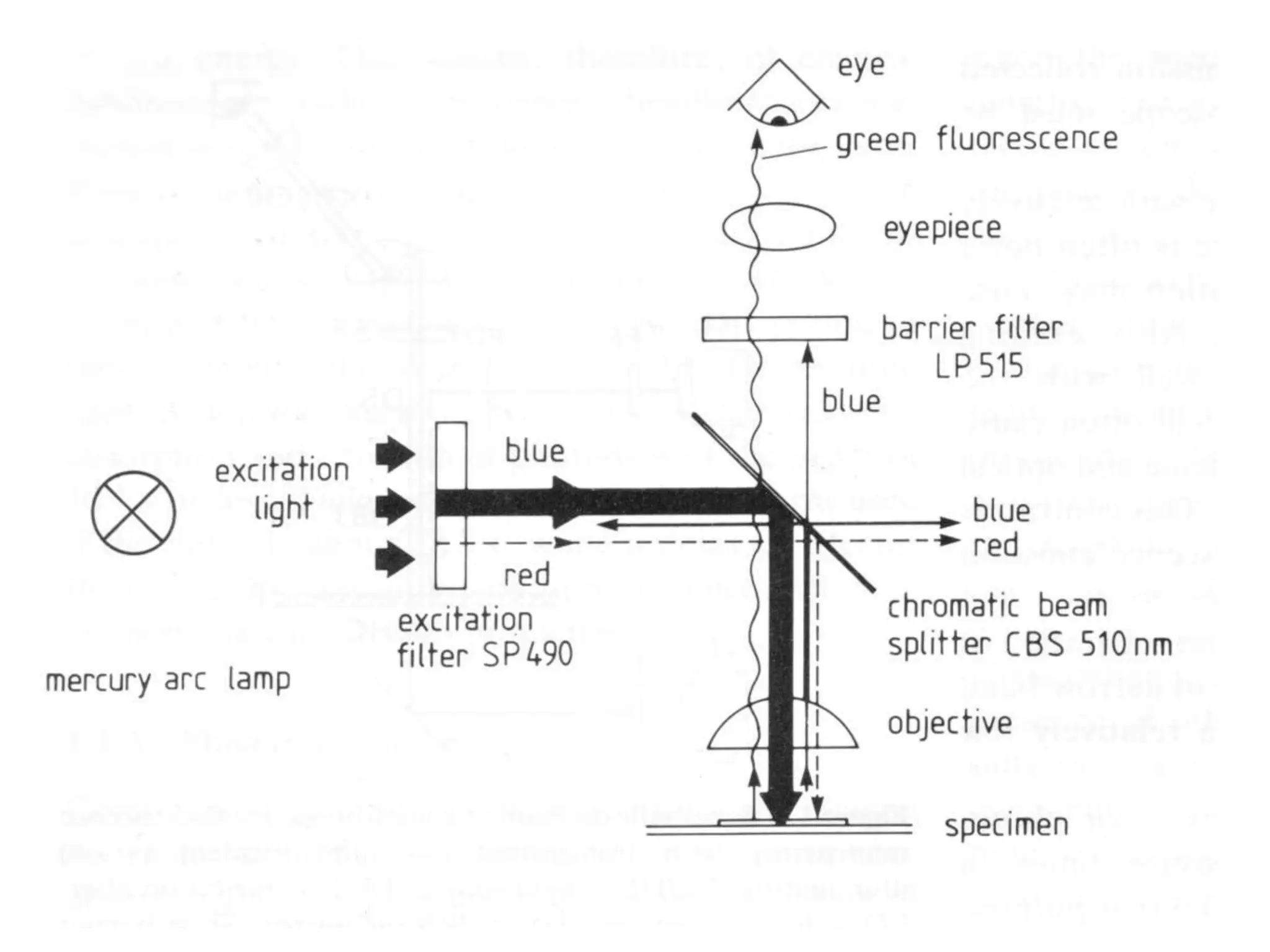
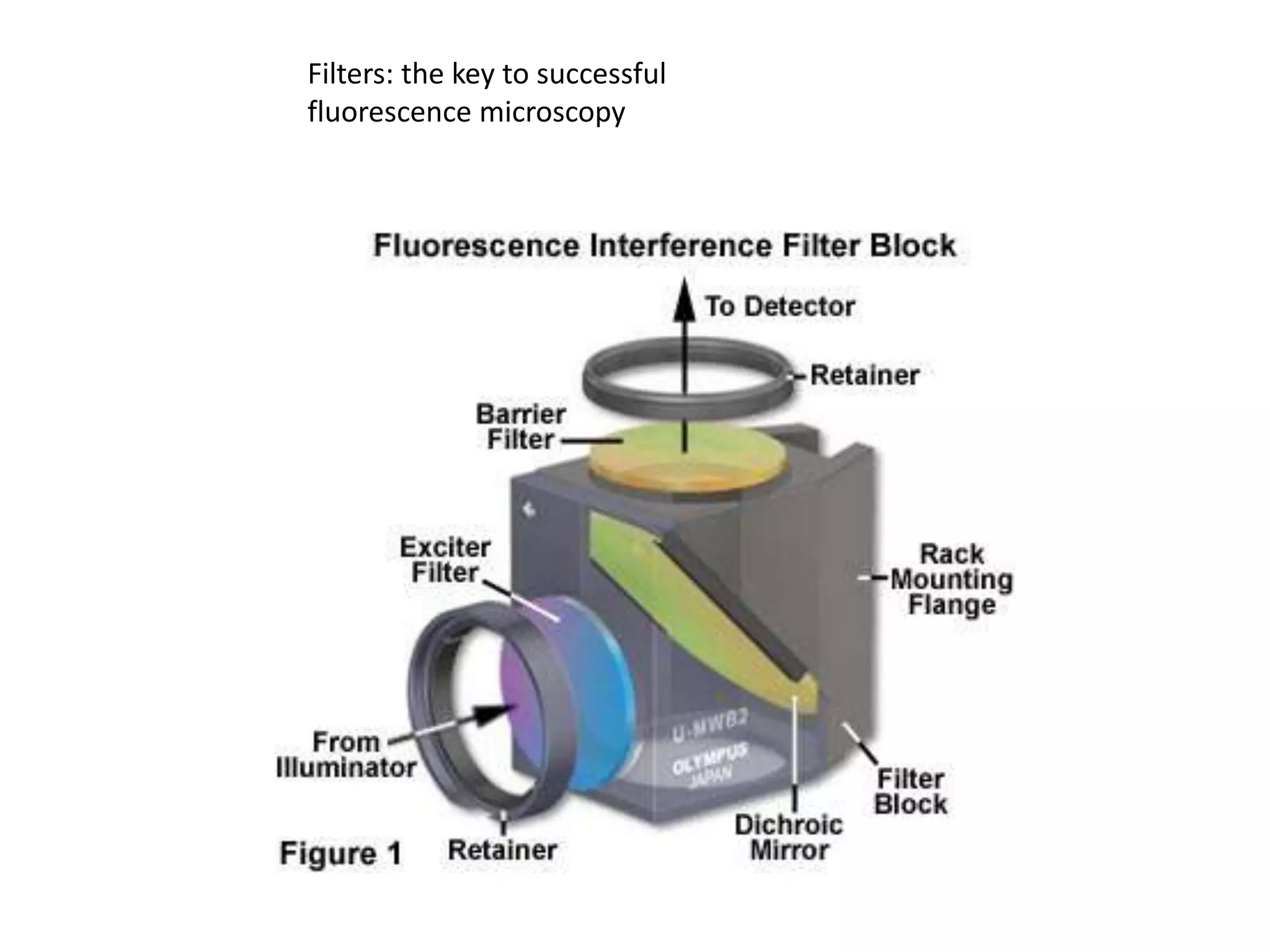
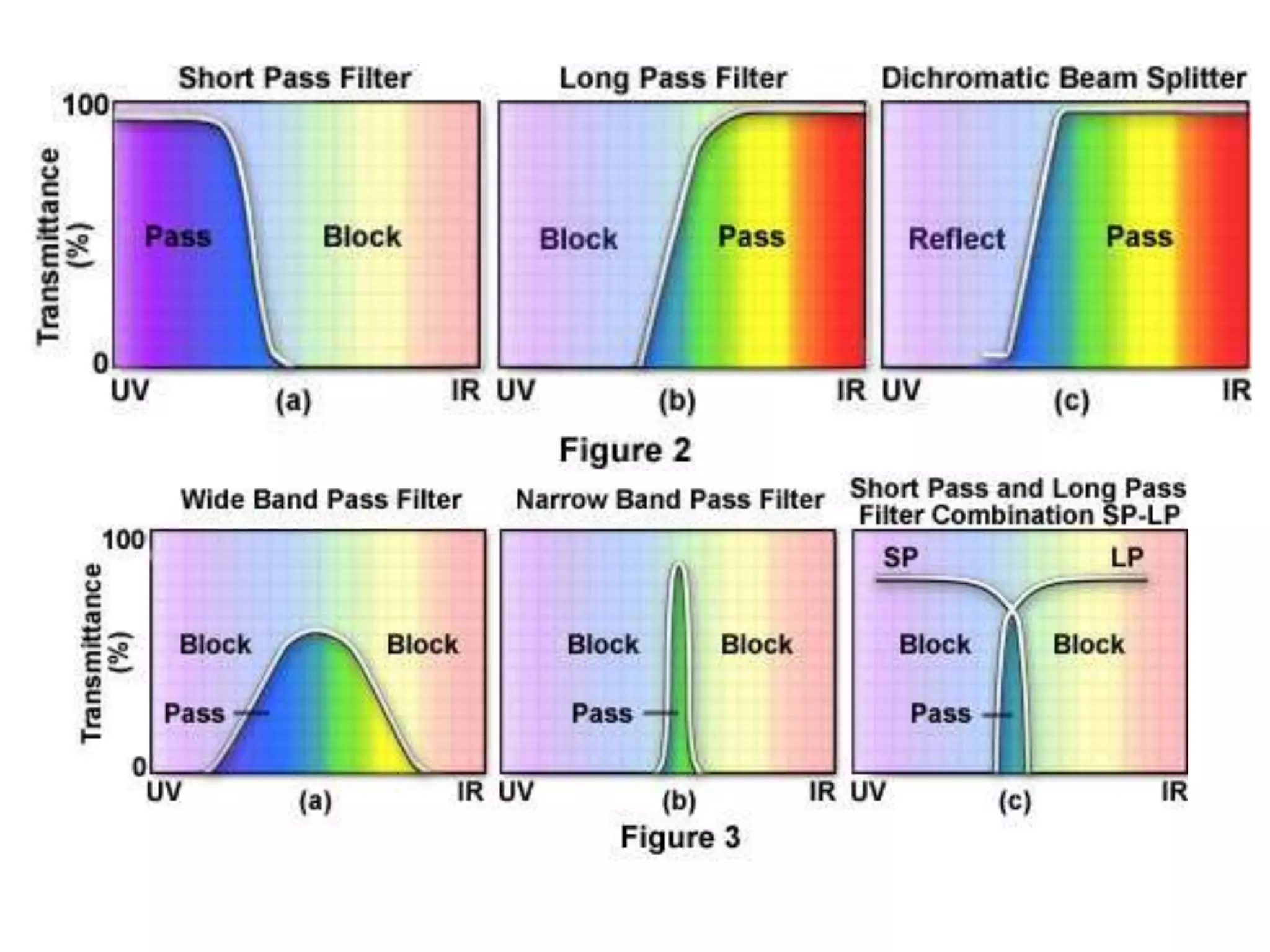
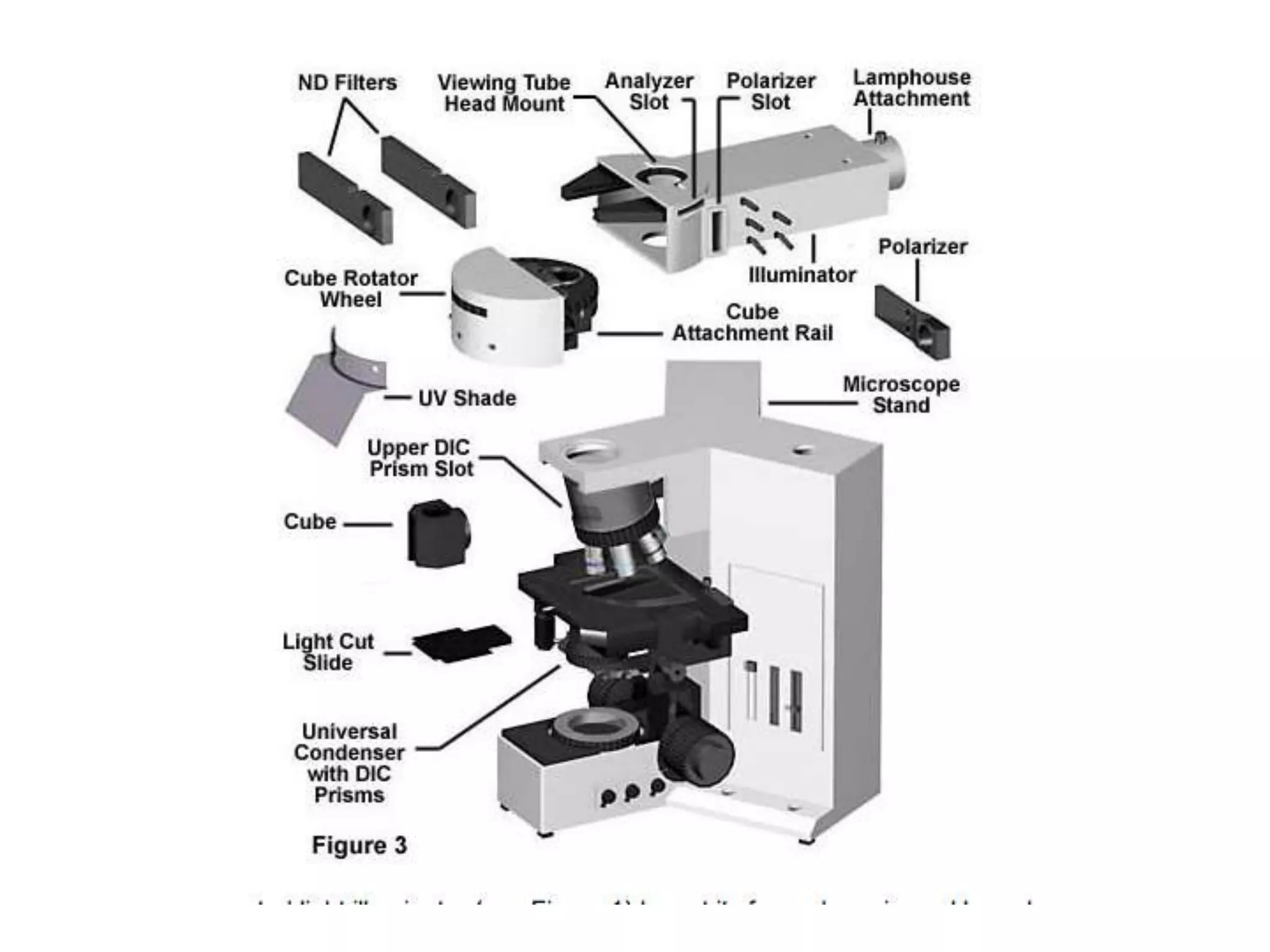
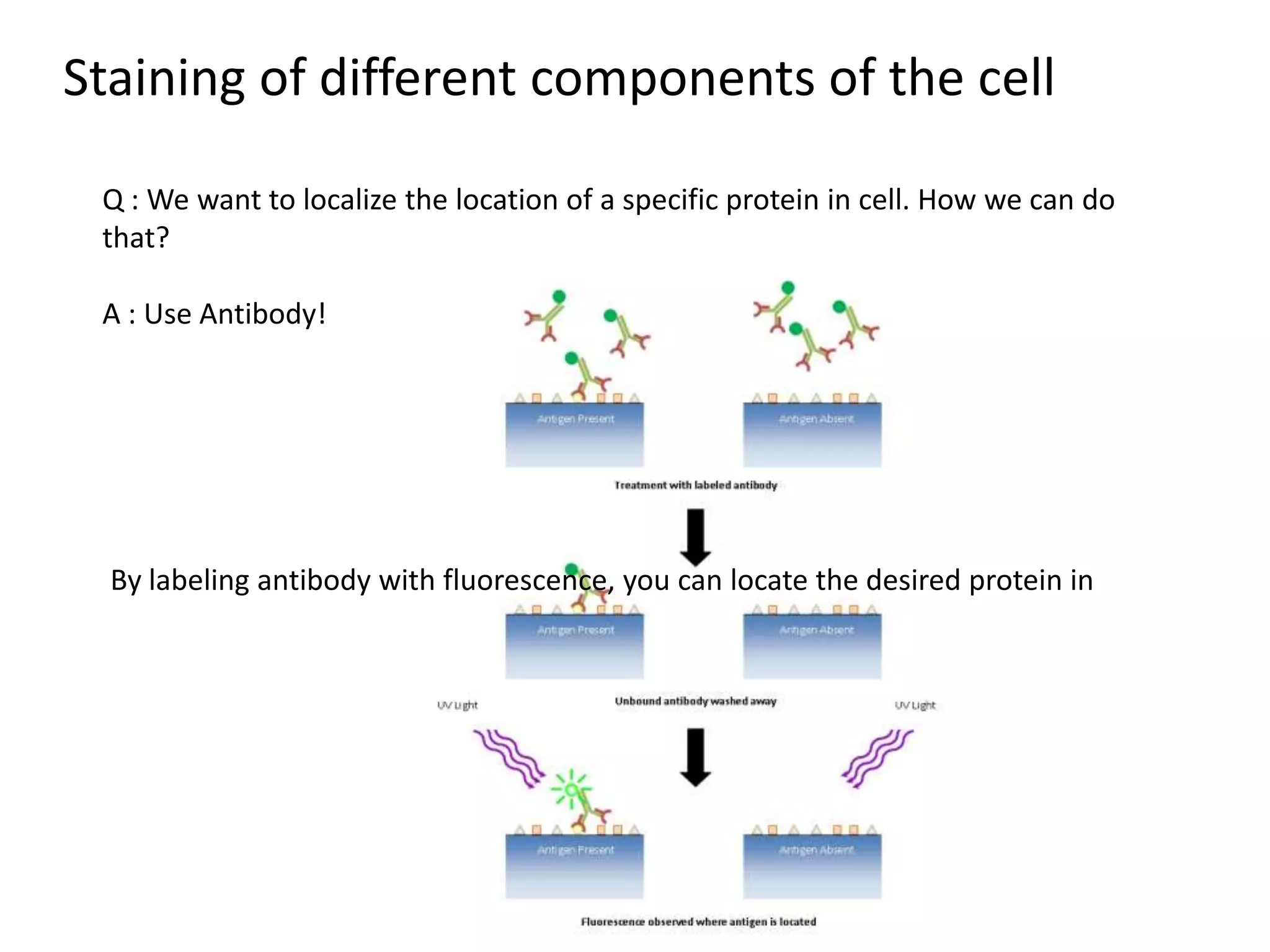
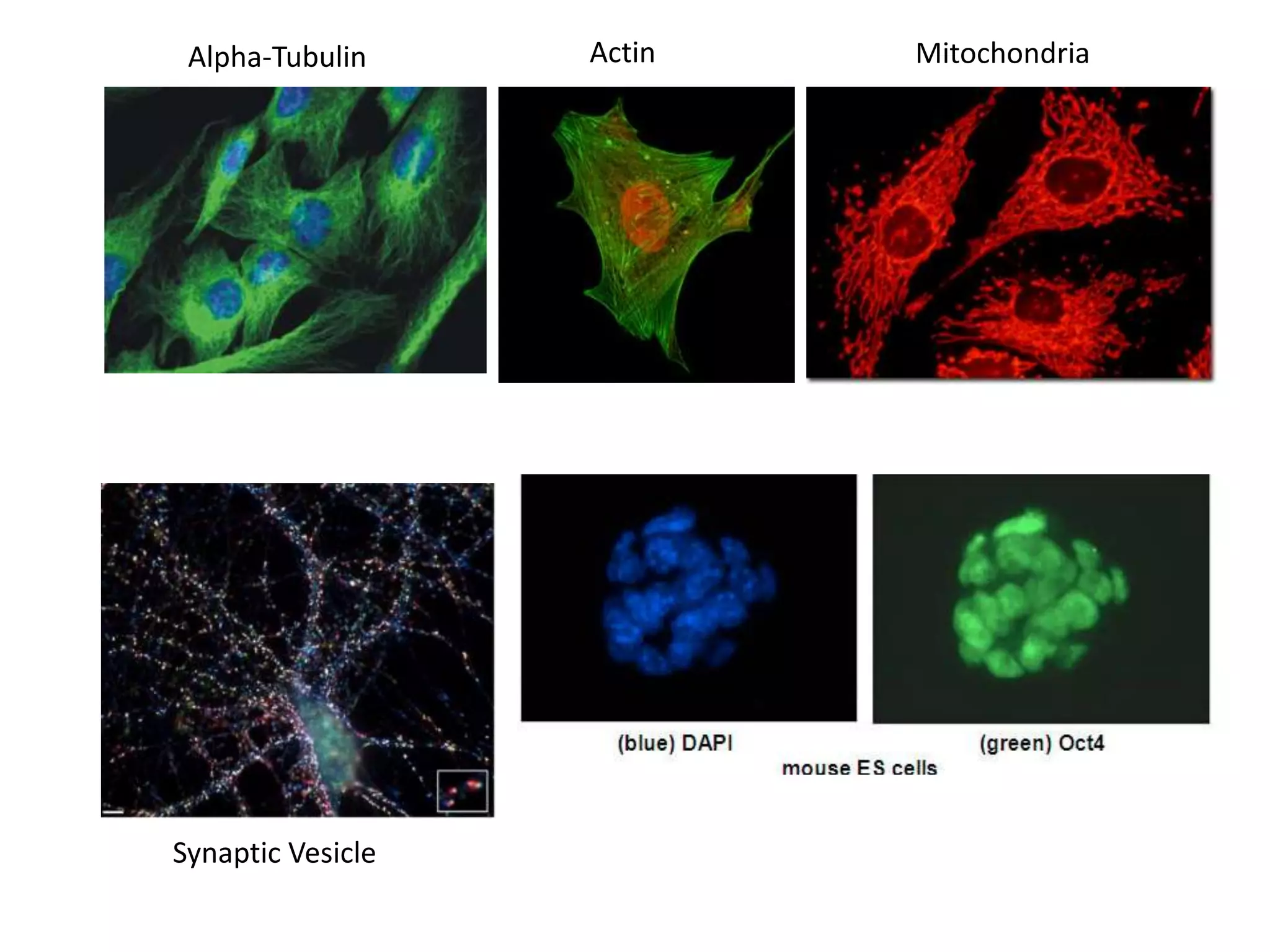
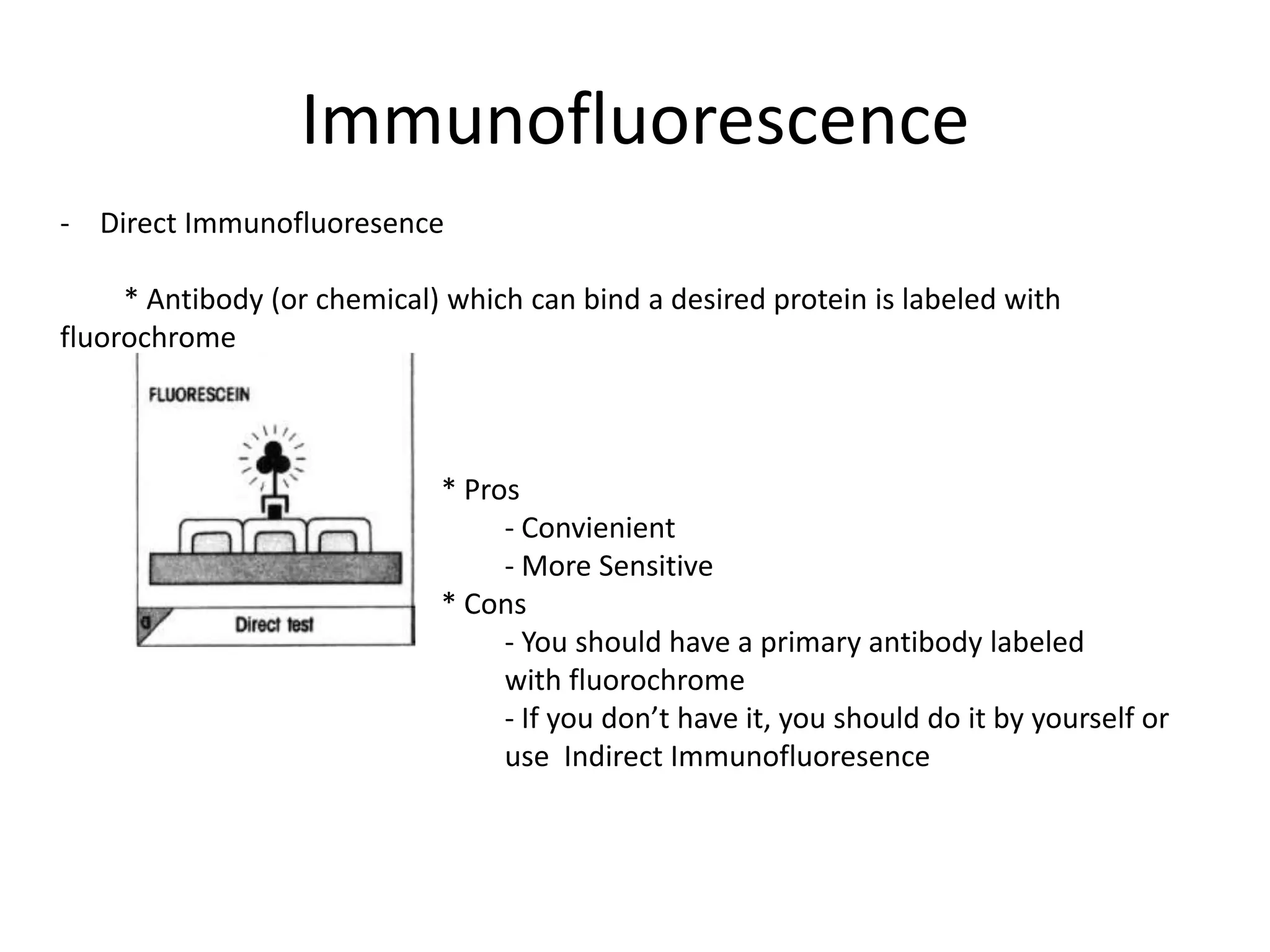
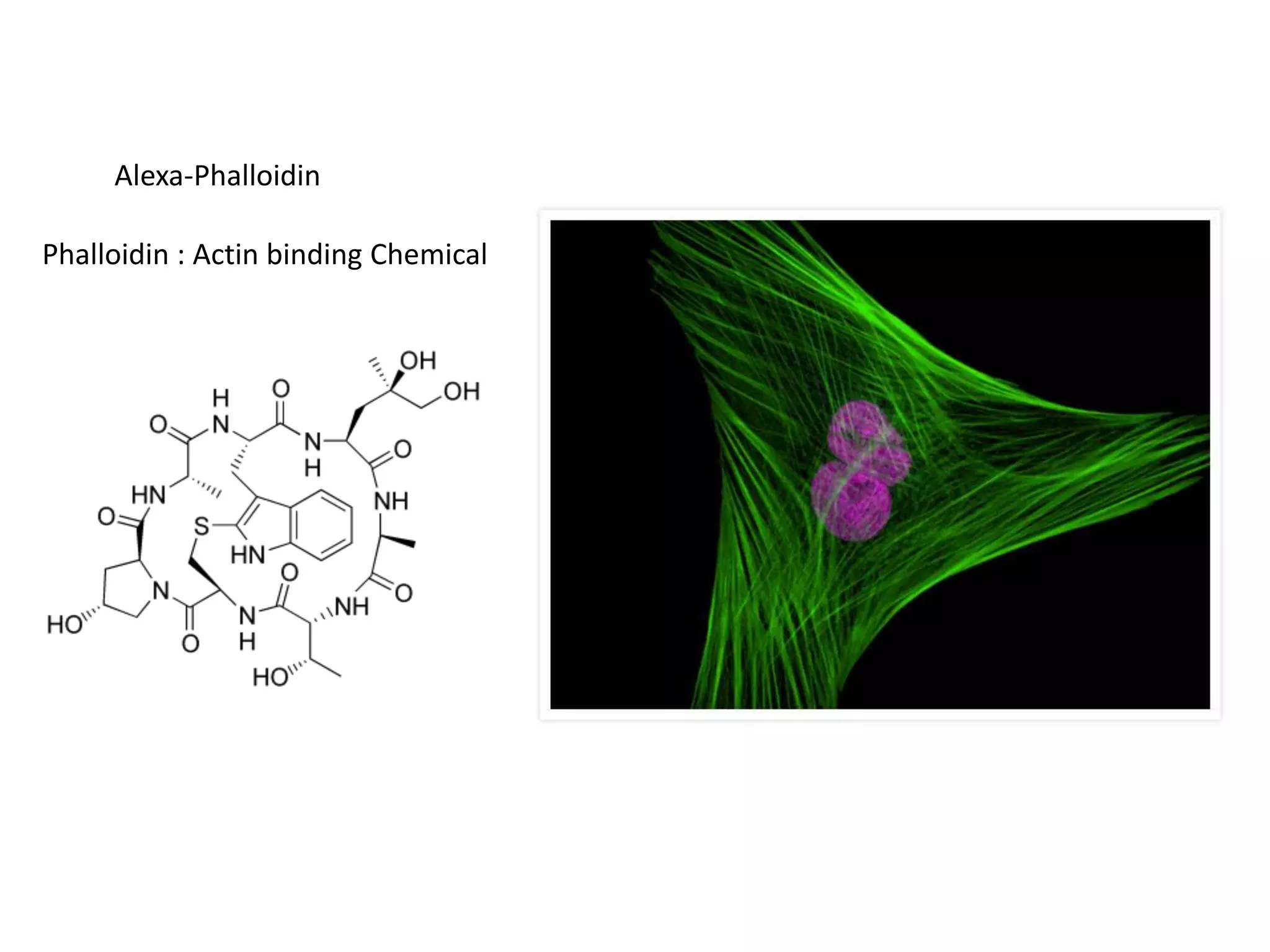
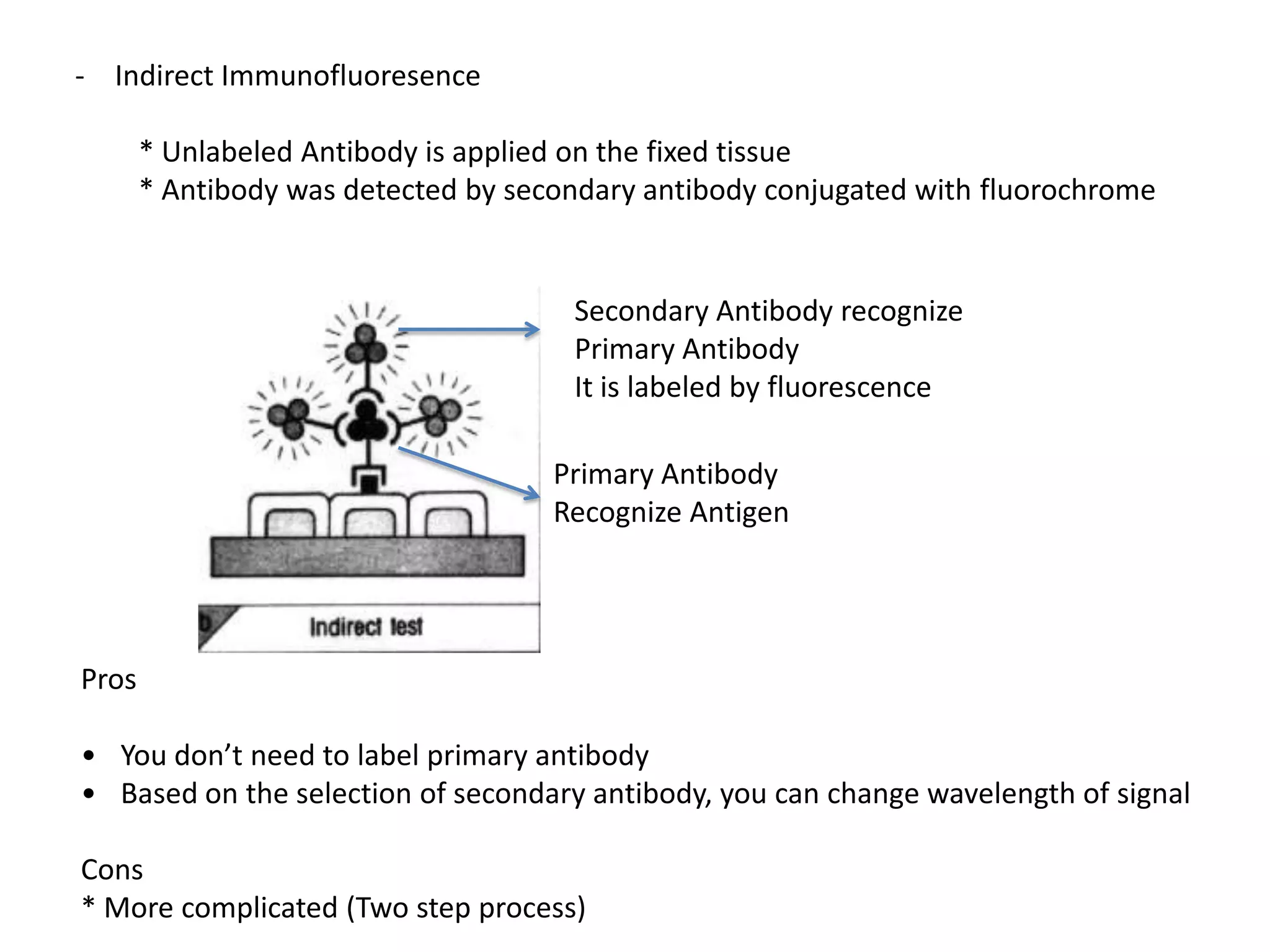
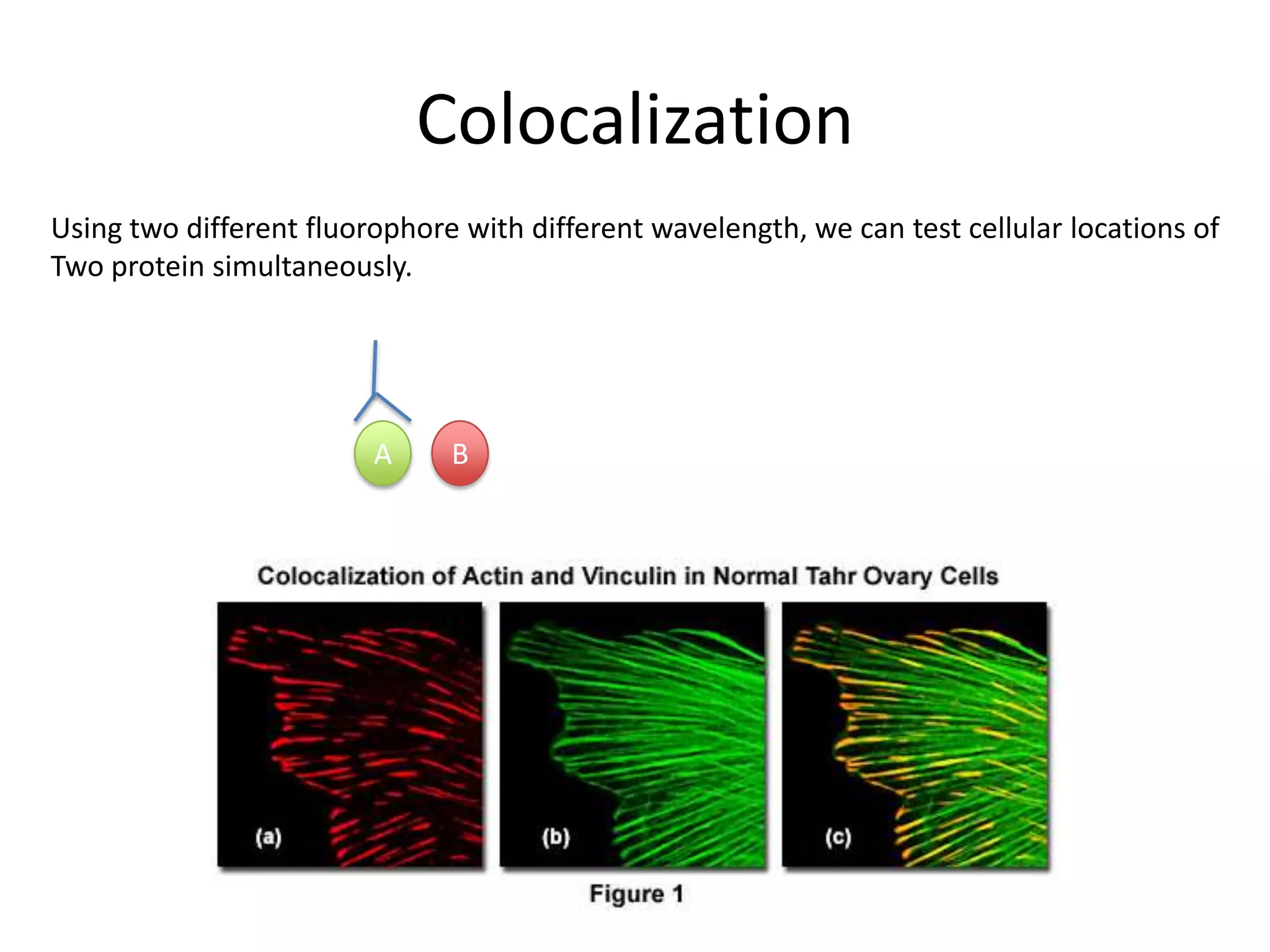
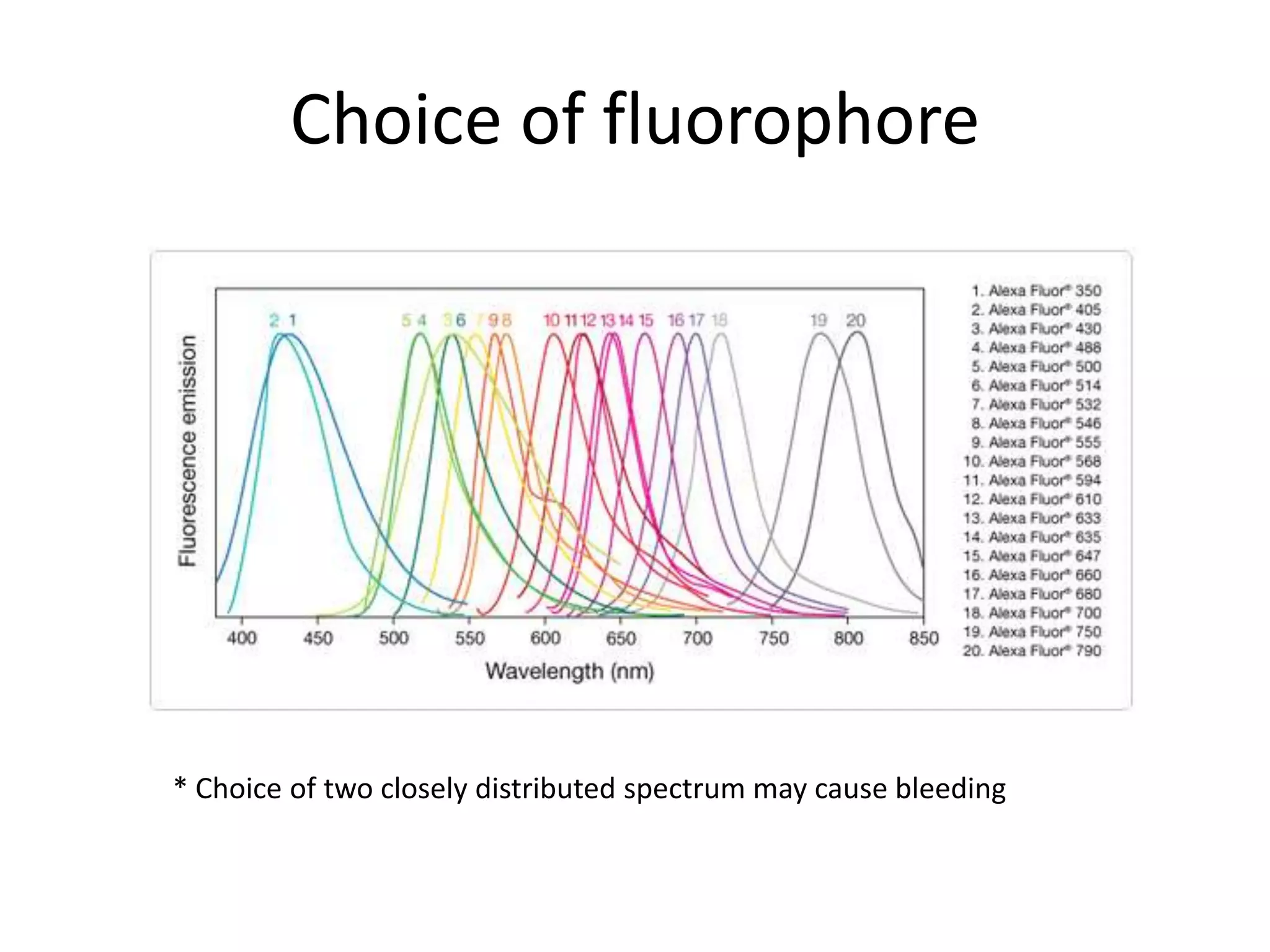
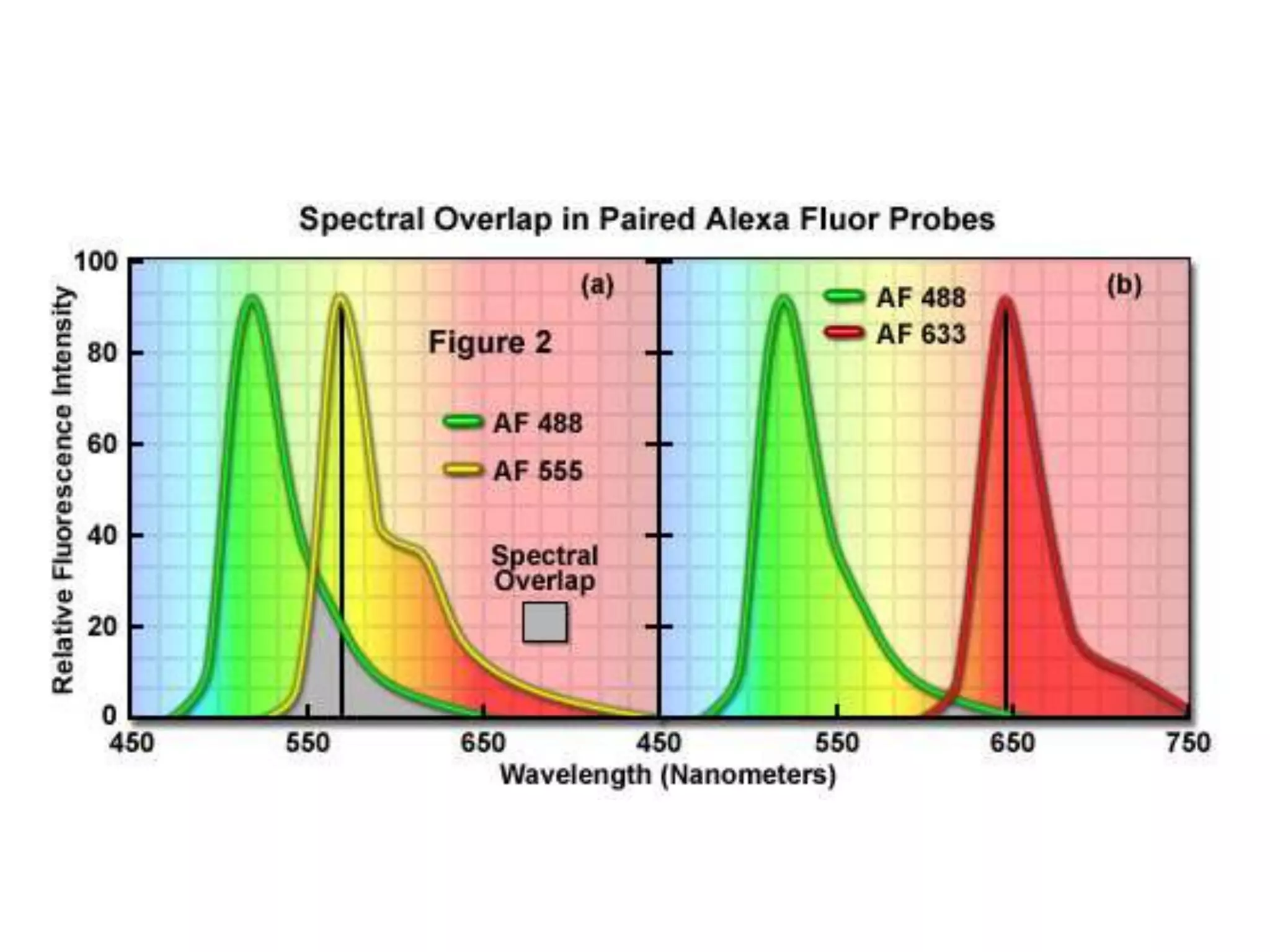
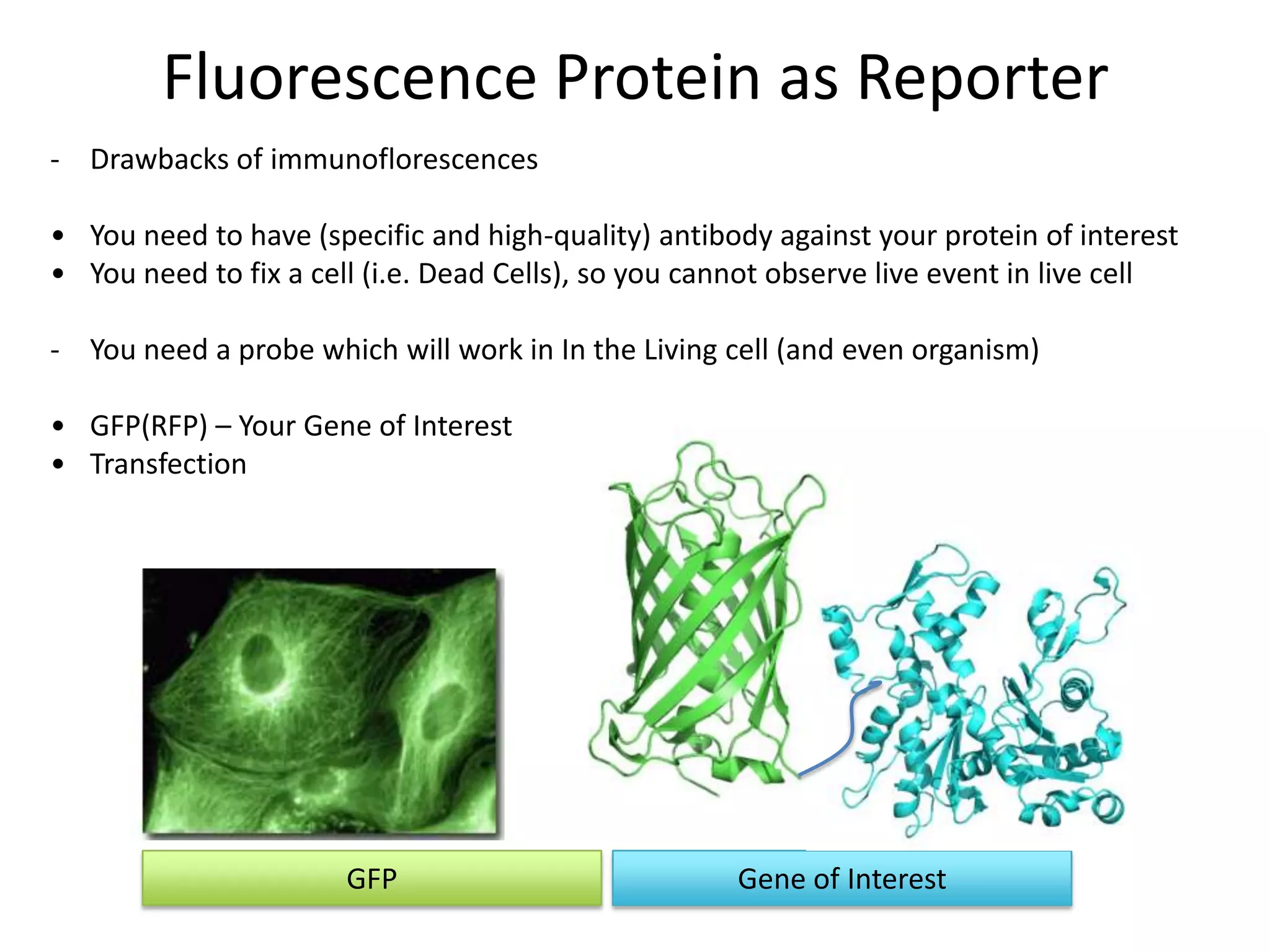
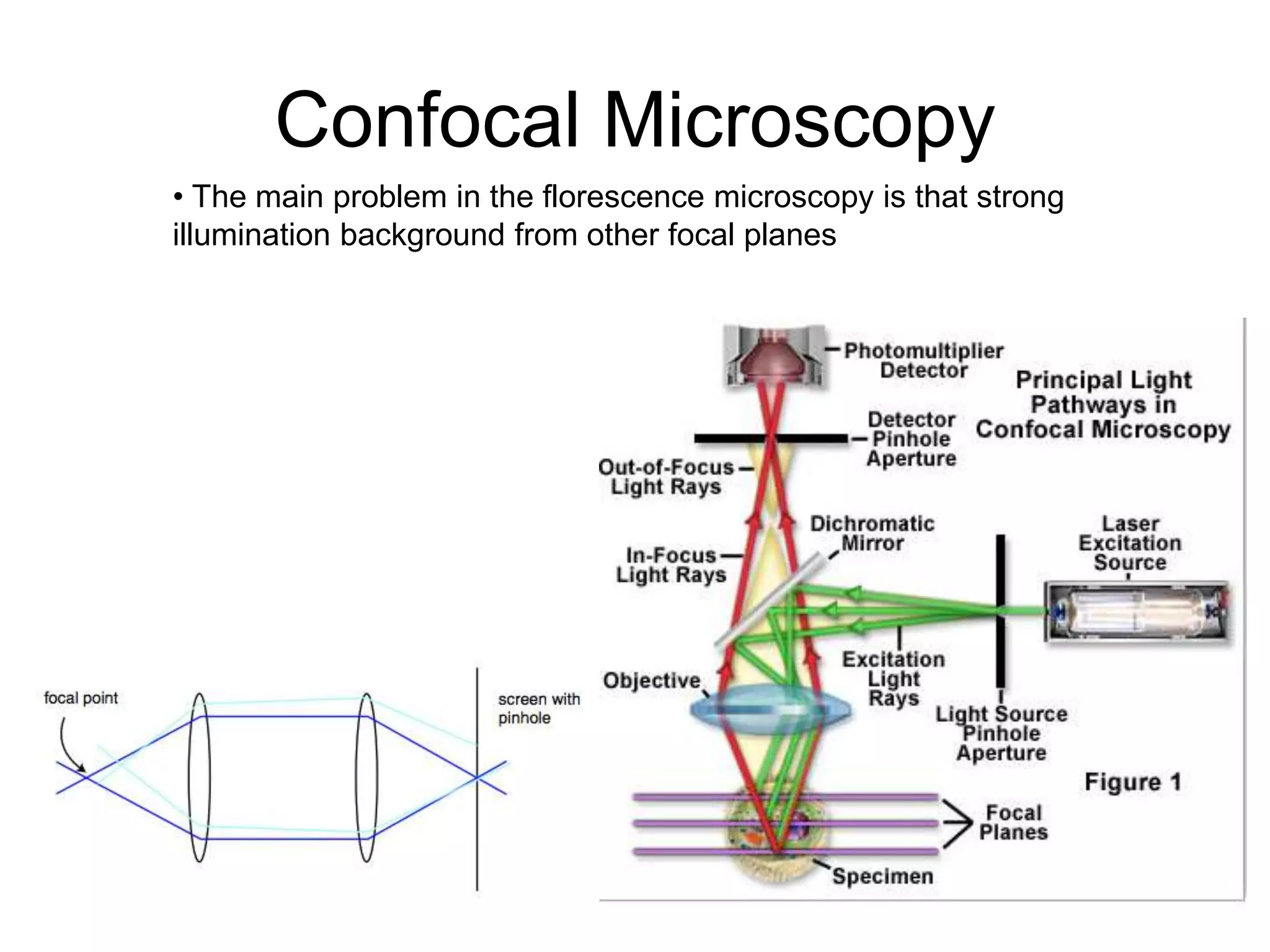
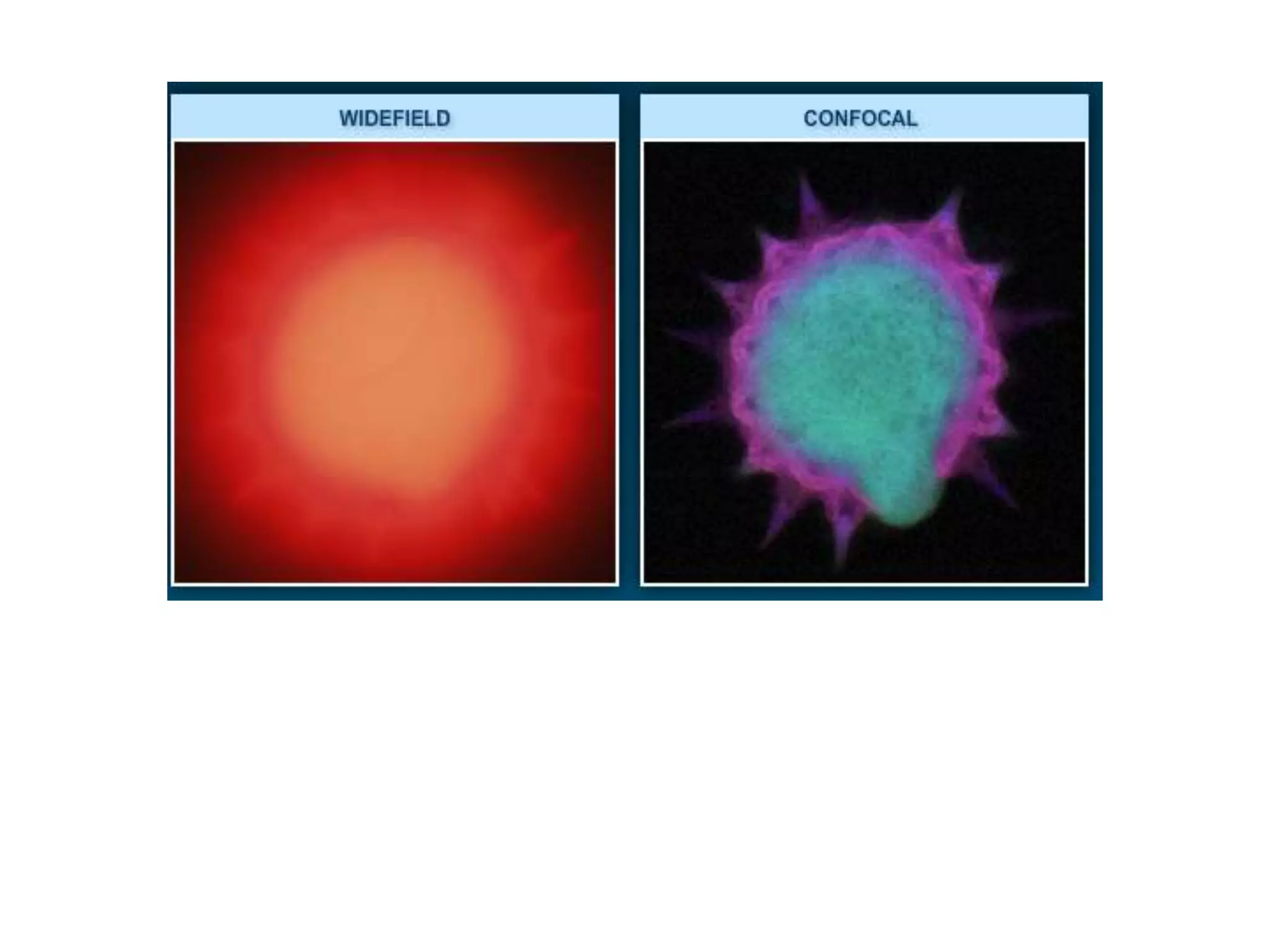
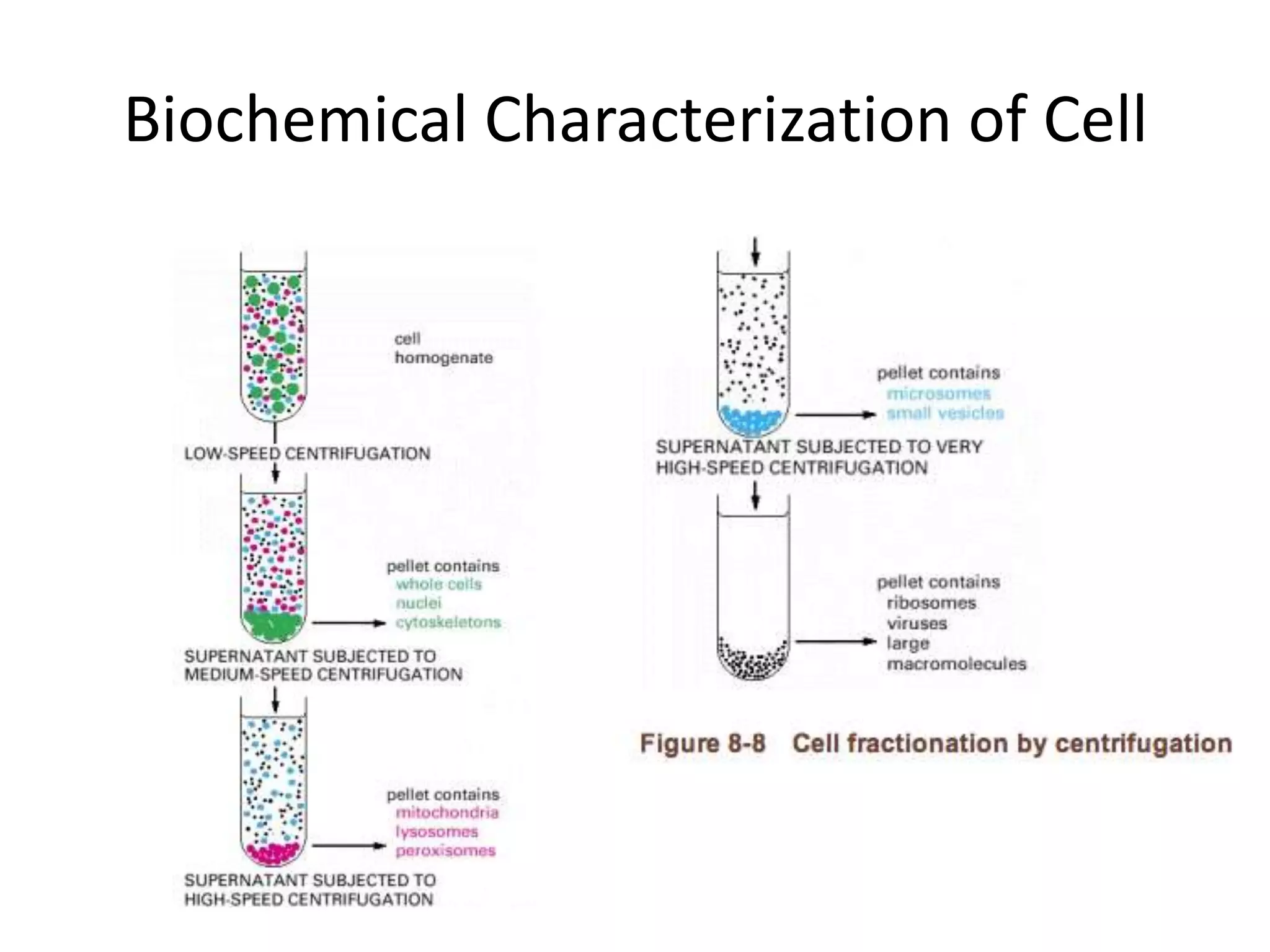
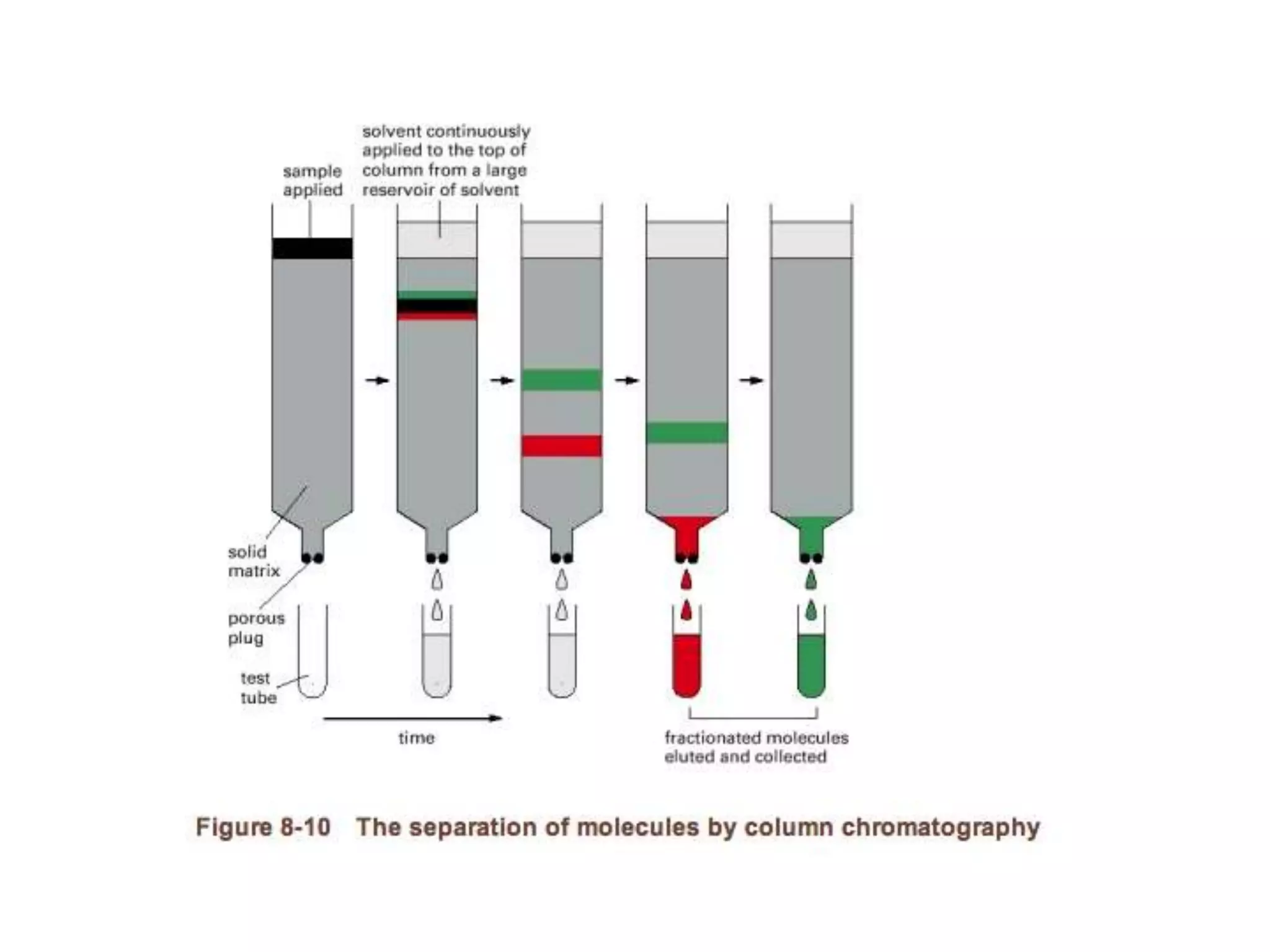
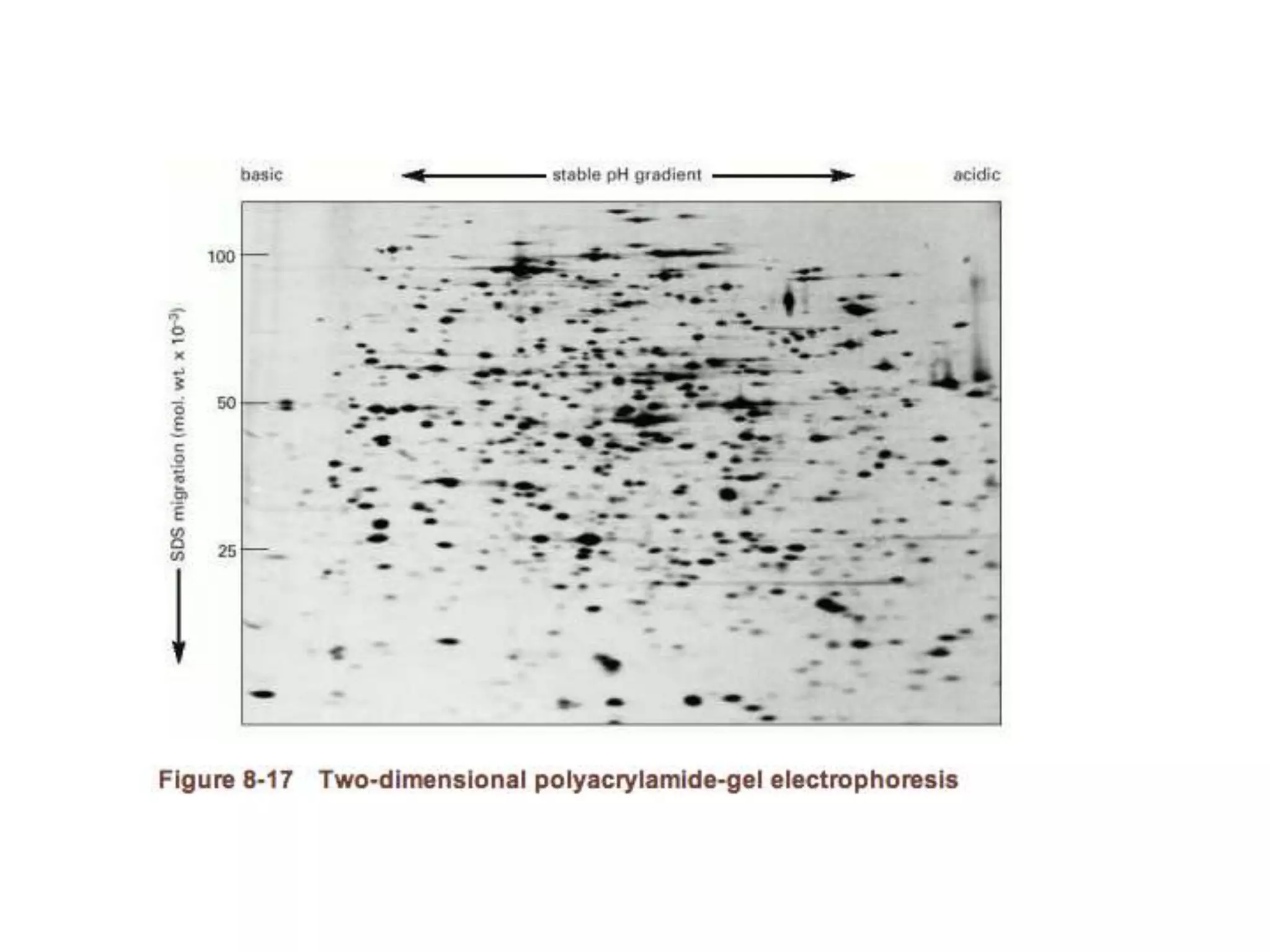
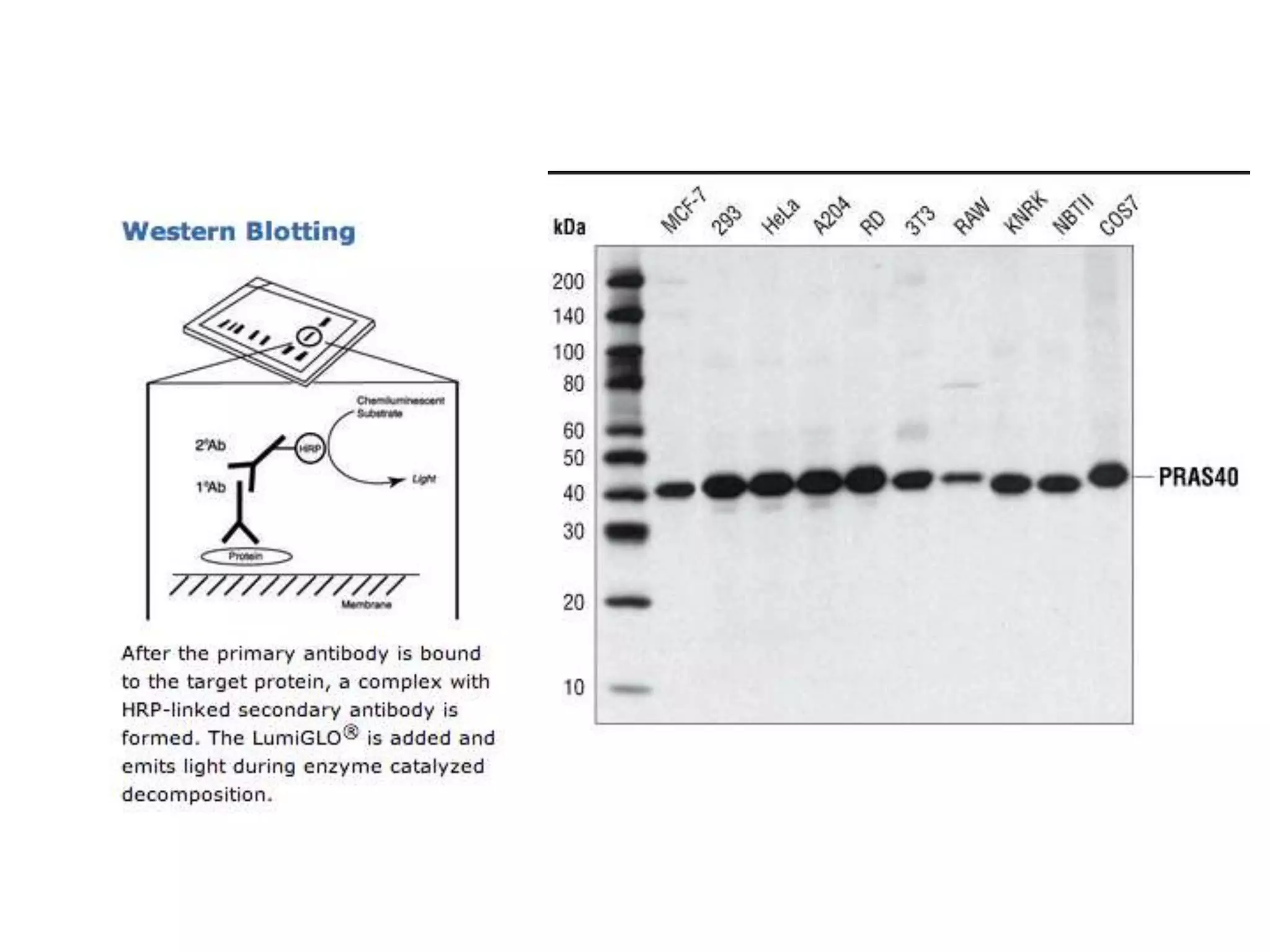
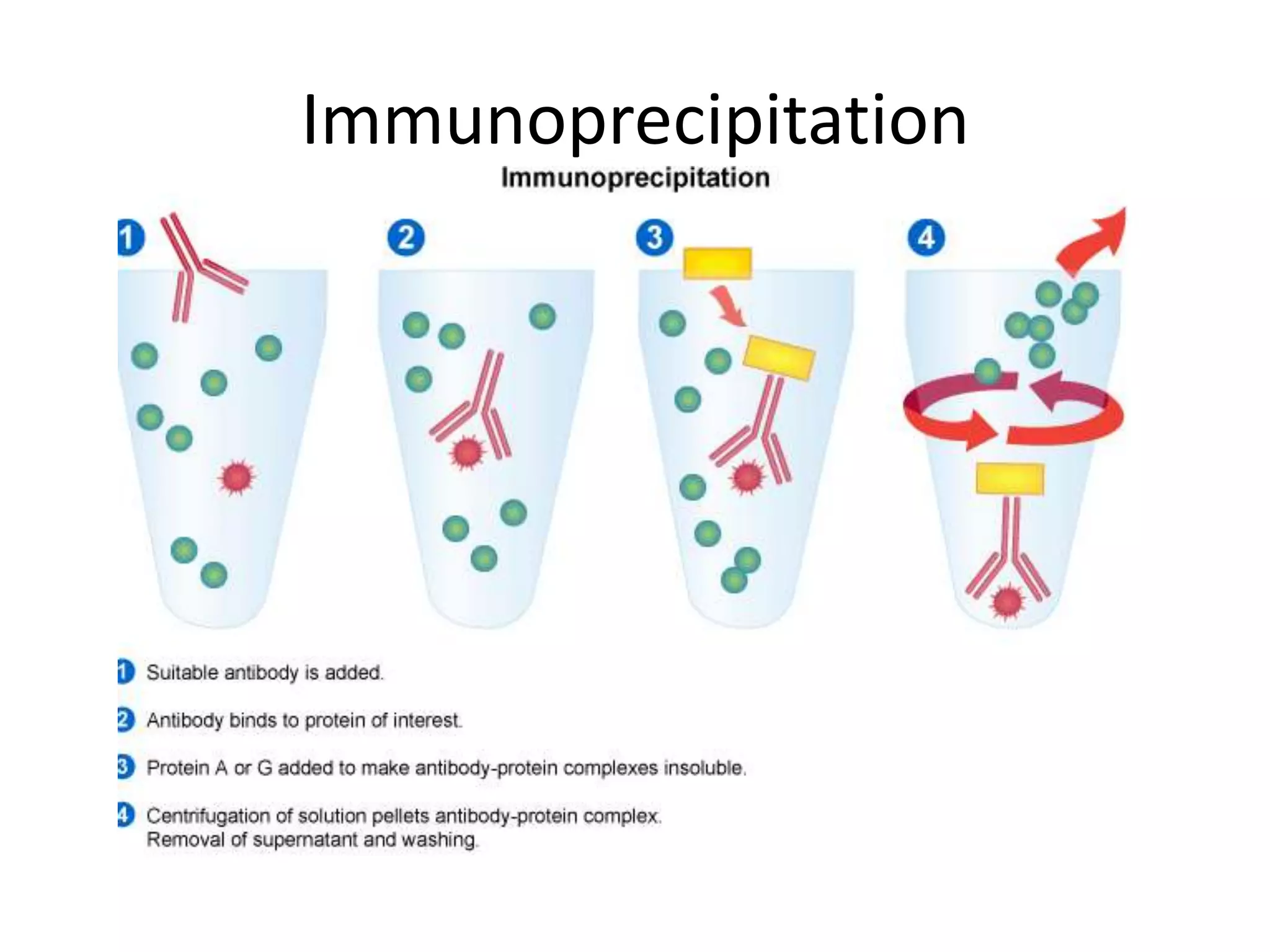
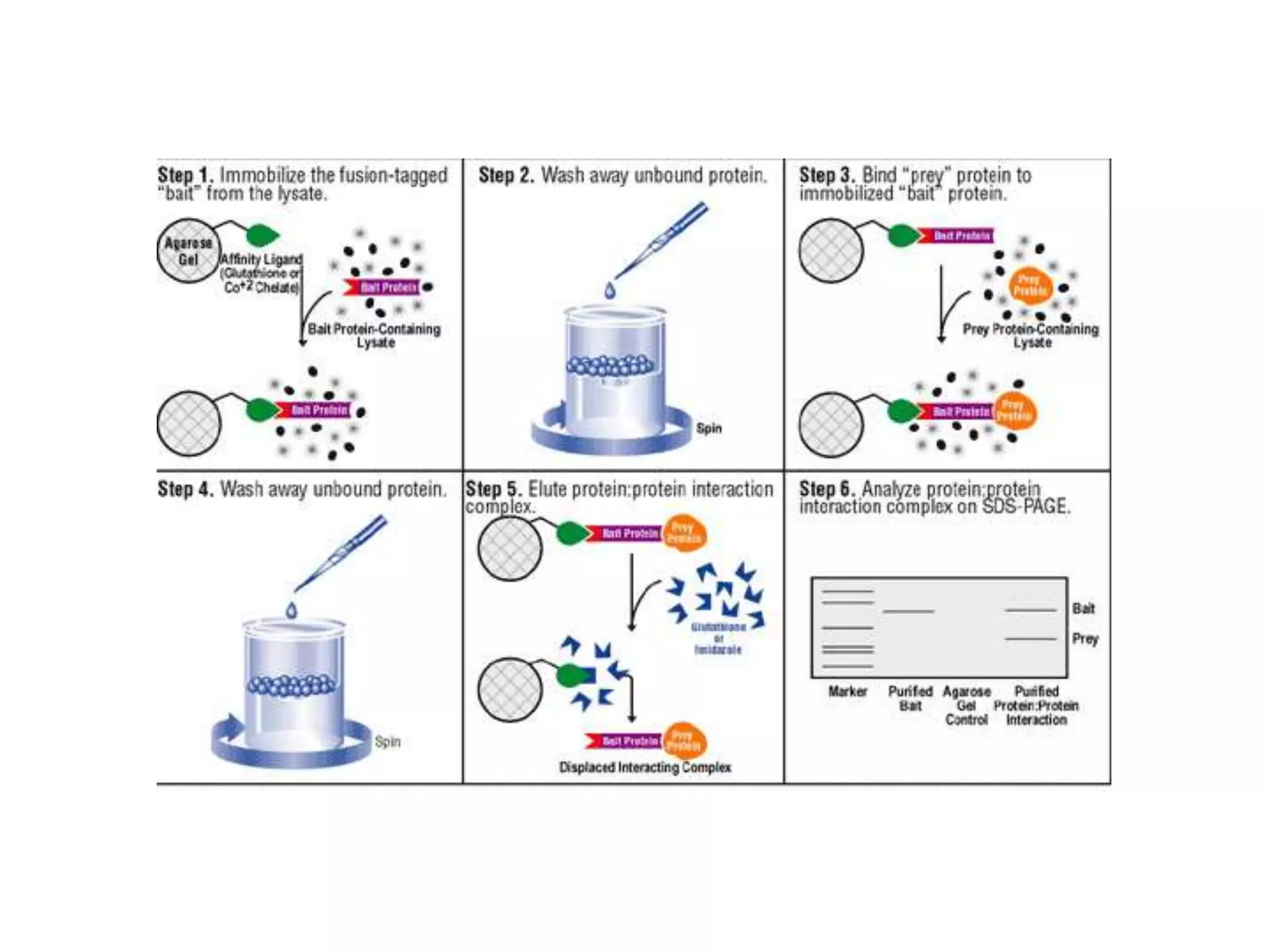
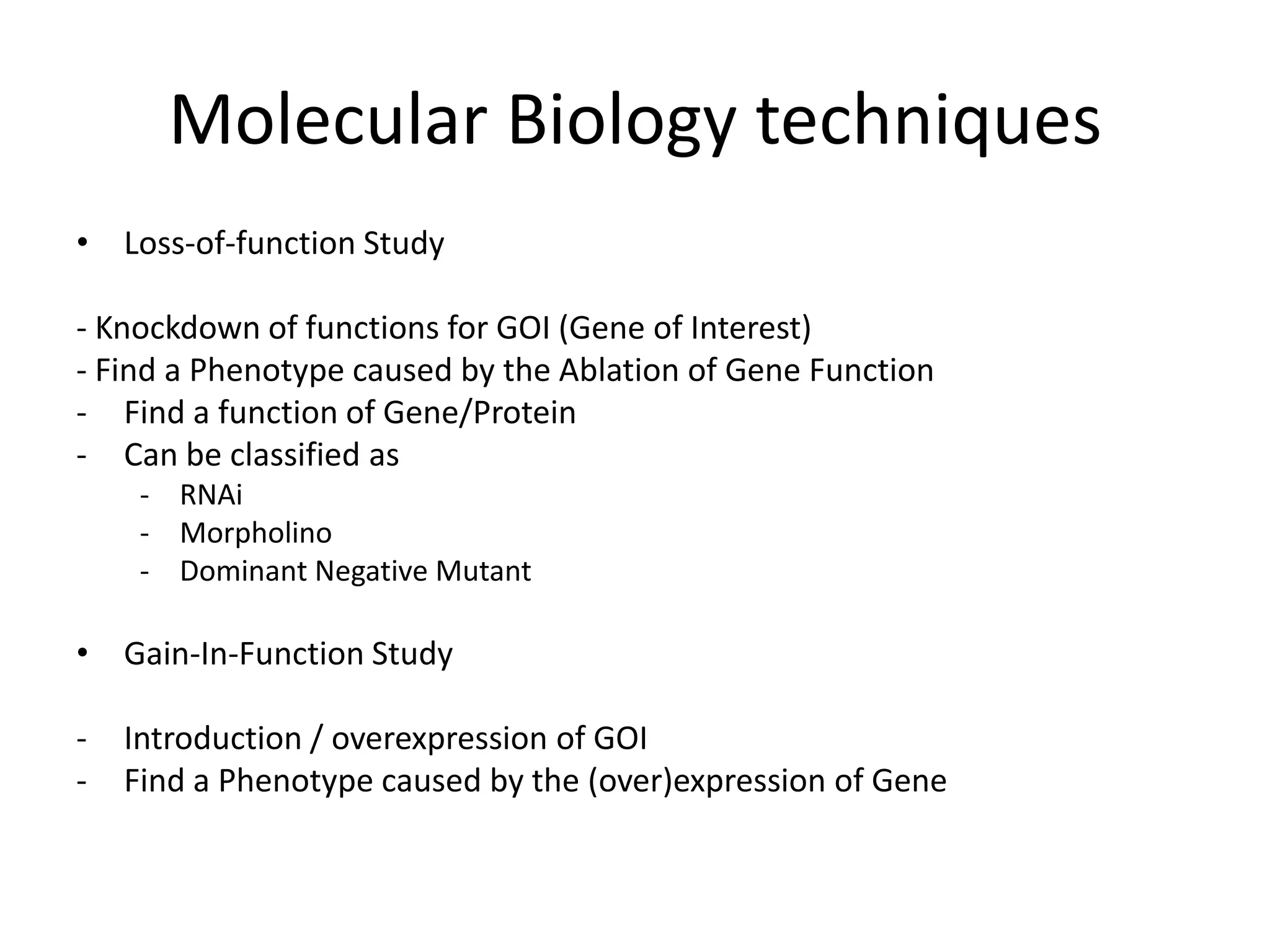
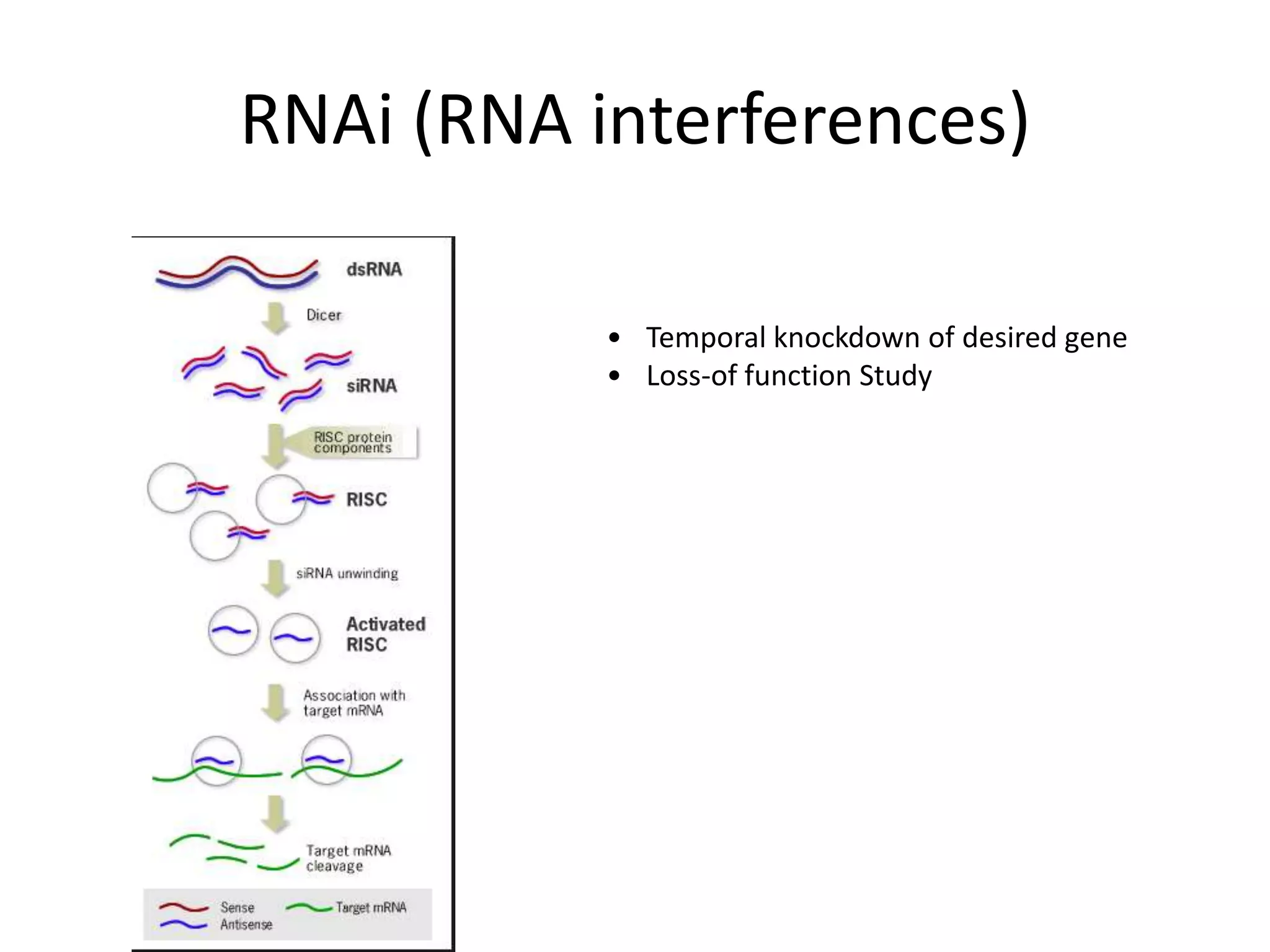
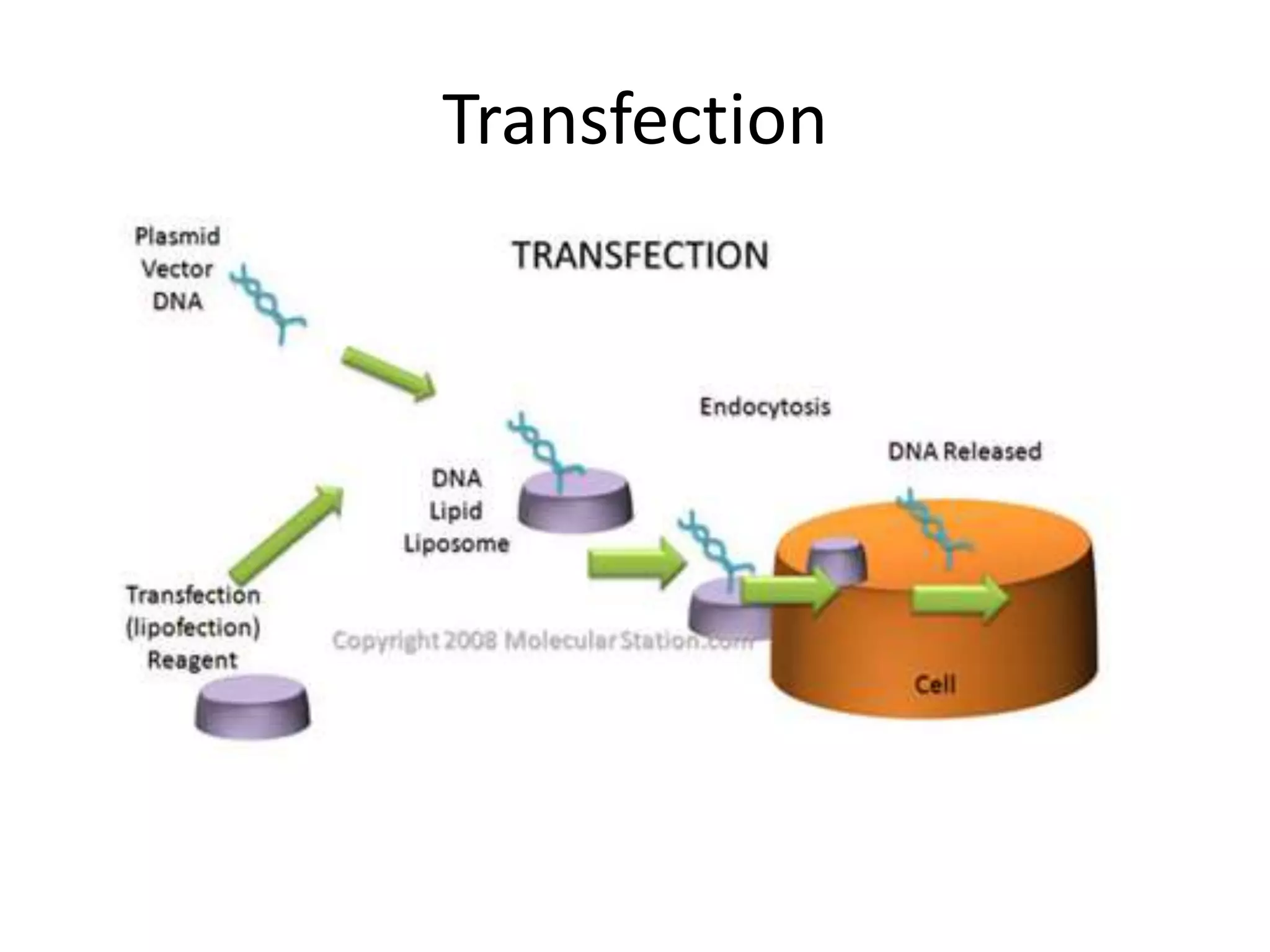
This document outlines the syllabus for an Advanced Animal Cell Culture course. It lists the topics that will be covered in each class, including introductions to cell culture, stem cells, transgenic and knockout animals, and genome engineering. It also provides details about some of the topics, such as commonly used cell lines and model organisms, cell culture techniques like subculture and cryopreservation, and research methods using cultured cells like microscopy, staining, and molecular biology techniques. The document emphasizes that cultured cells can serve as useful model systems to study specific cell types and biological phenomena while avoiding complications from whole organisms.