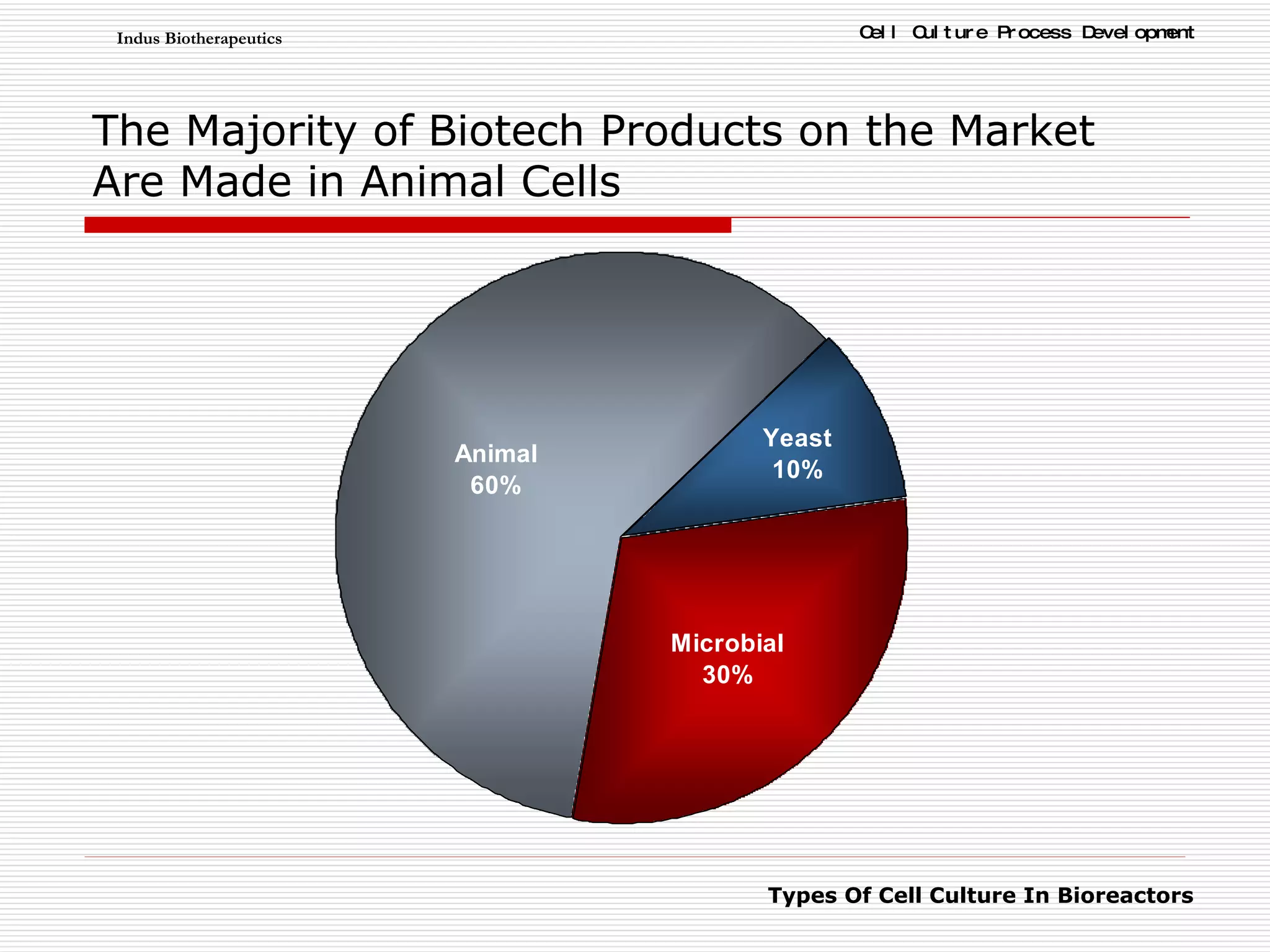
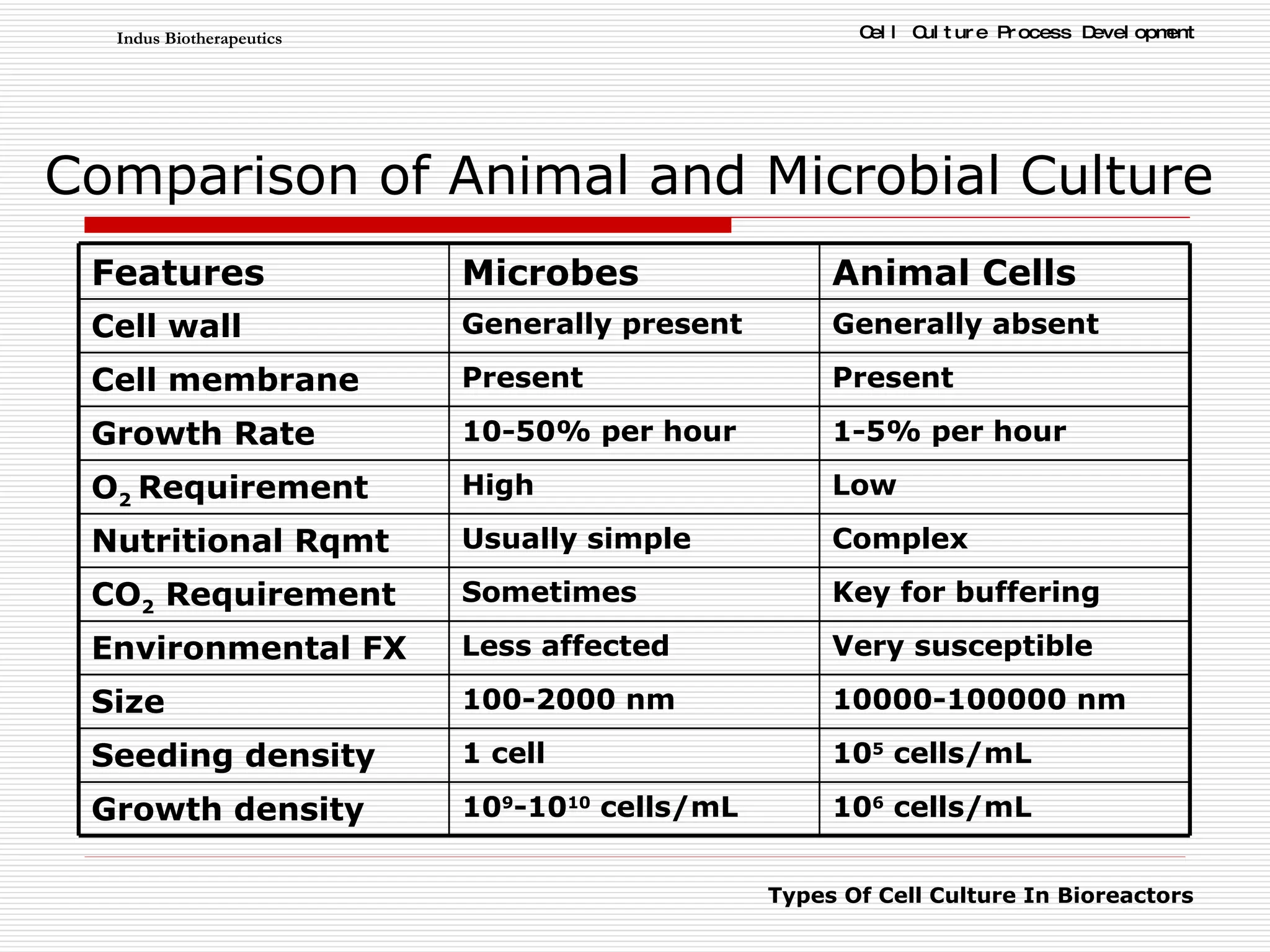
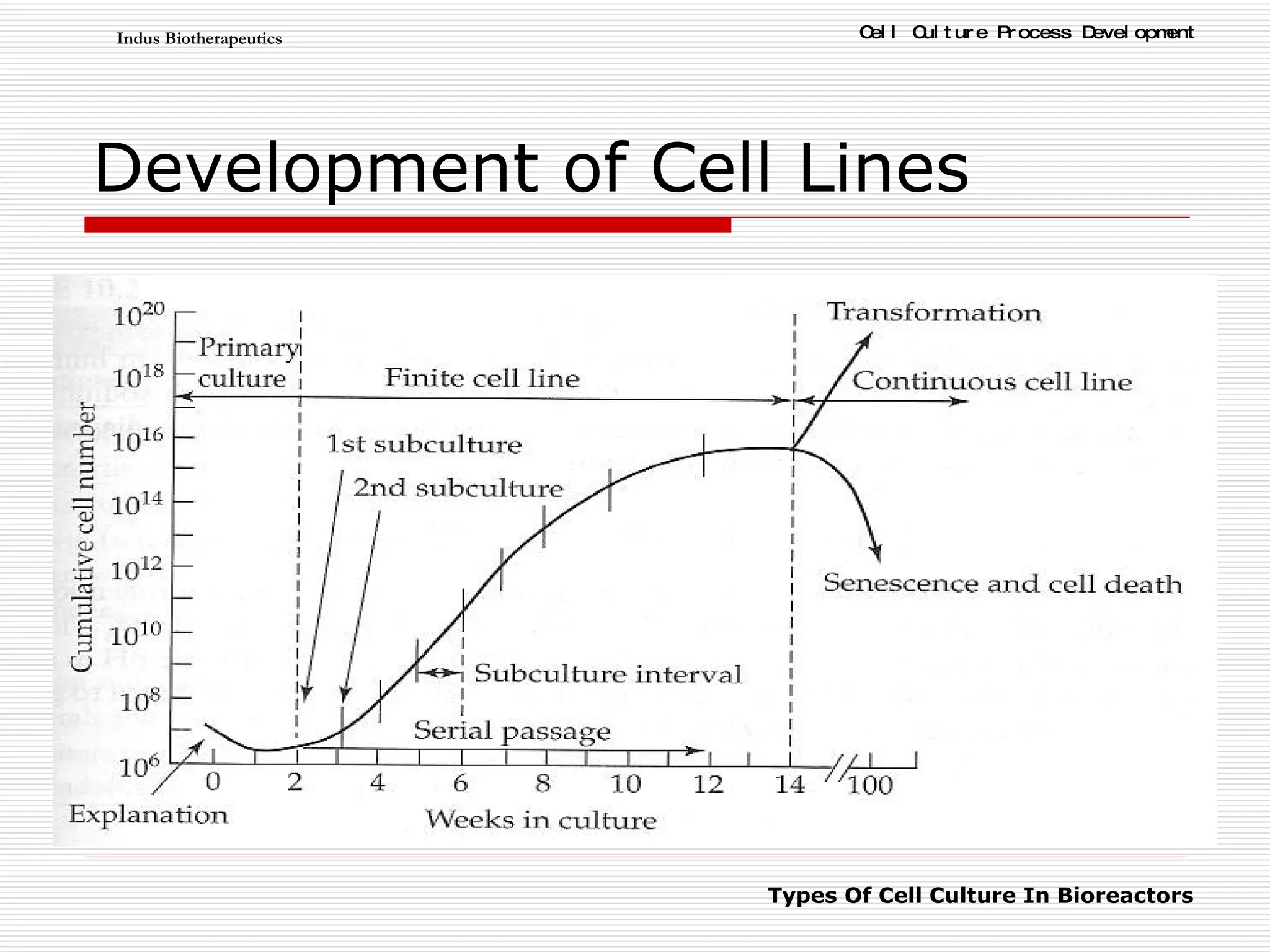
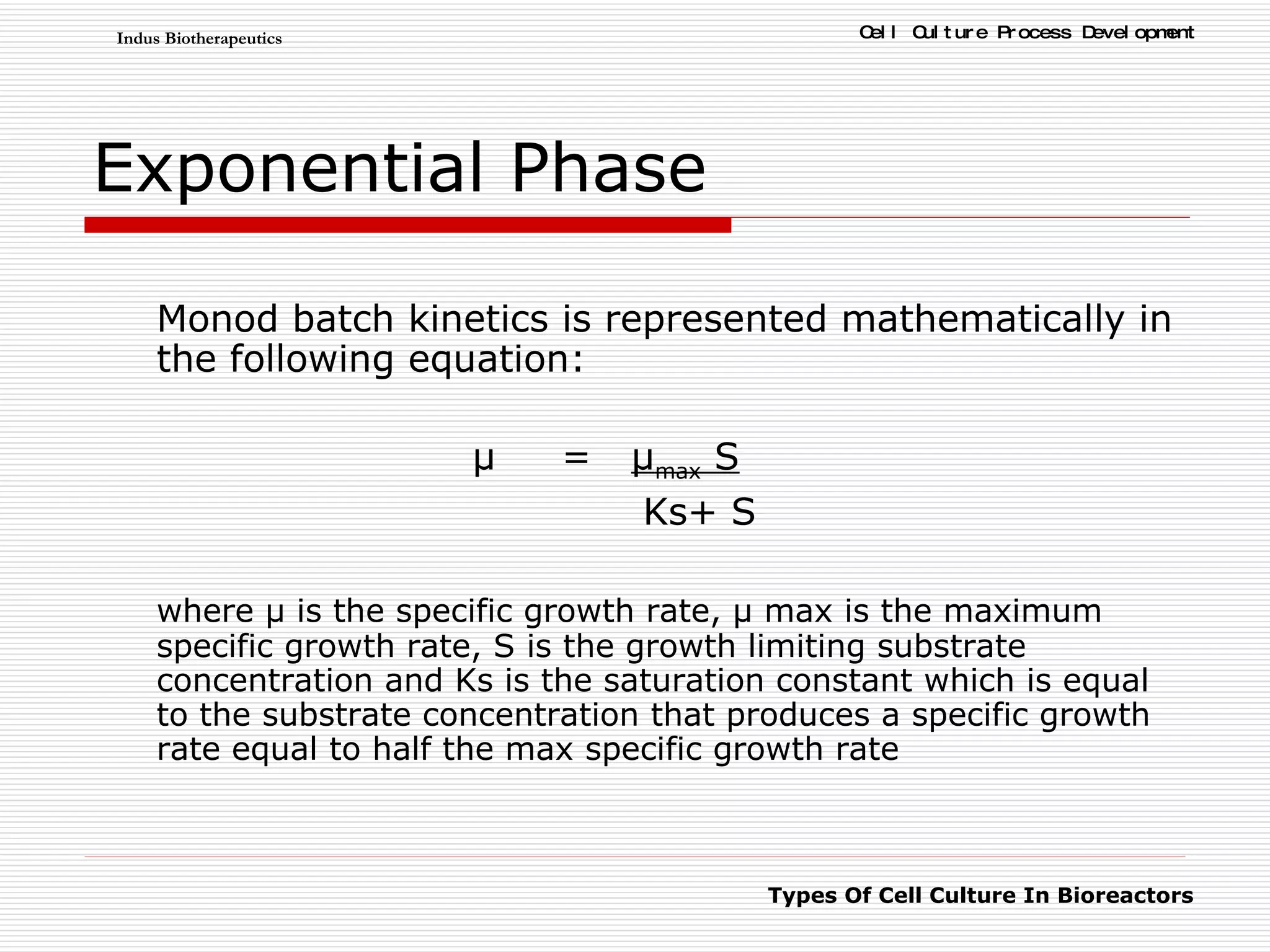
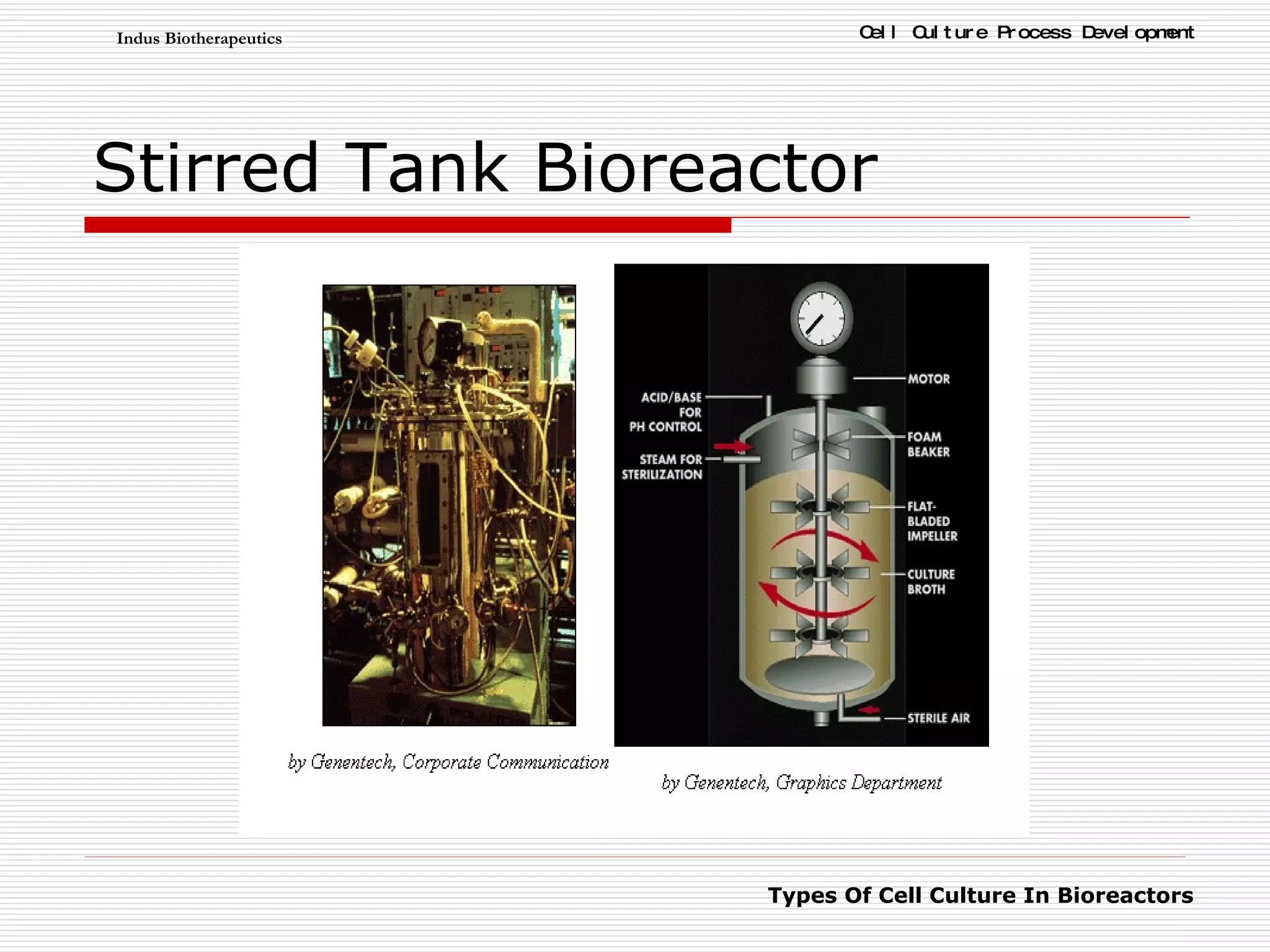
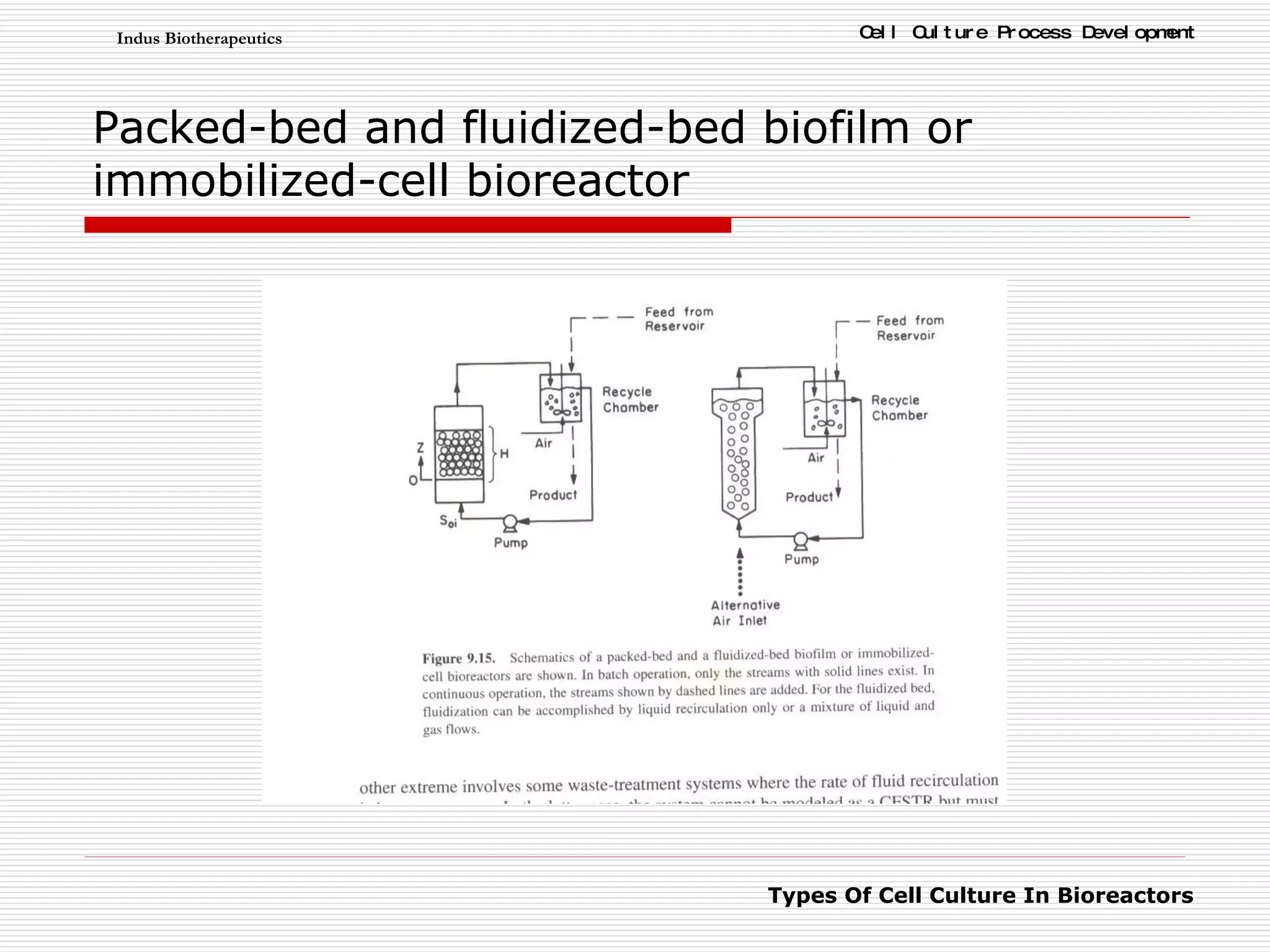
The document discusses different types of cell culture used in bioreactors. It describes organ culture, tissue culture, and cell culture. Cell culture involves dispersing tissue enzymatically into a cell suspension that can be grown as a monolayer or in suspension. Continuous cell lines can be propagated indefinitely and have gained immortality through transformation. Bioreactors must provide a well-controlled environment for cell culture and can operate in batch, fed-batch or perfusion modes. Common bioreactor designs include stirred tank, airlift and wave bioreactors.