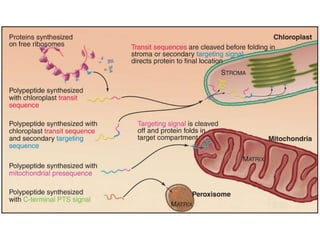
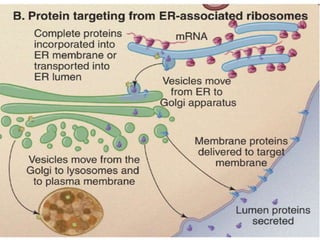
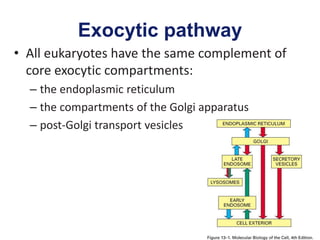
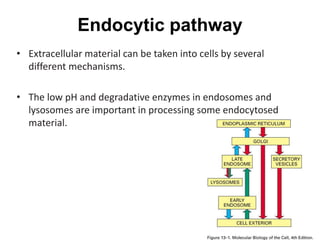
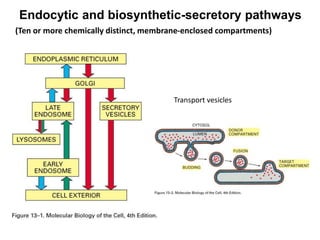
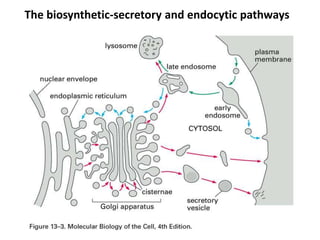
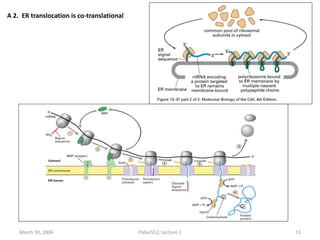
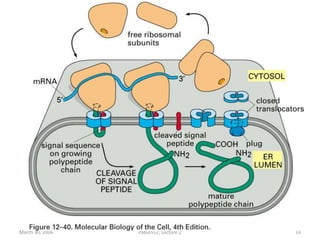
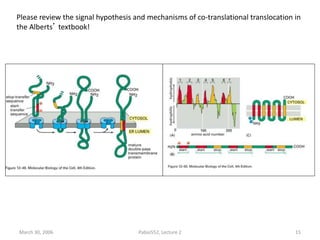
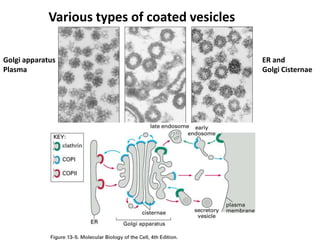
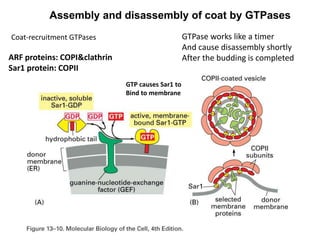
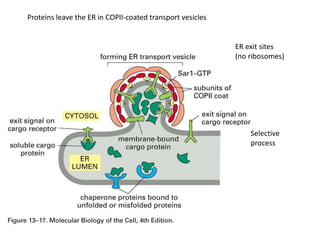
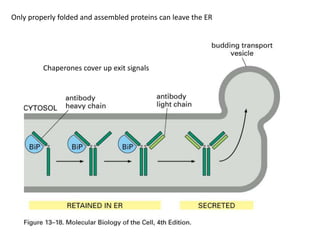
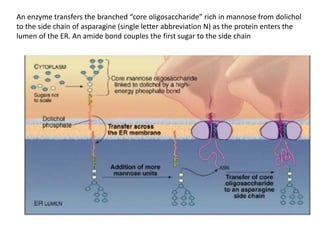
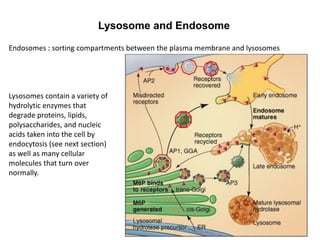
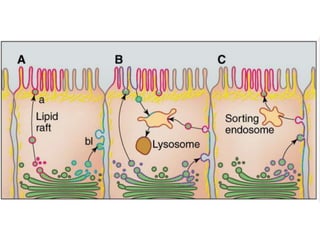
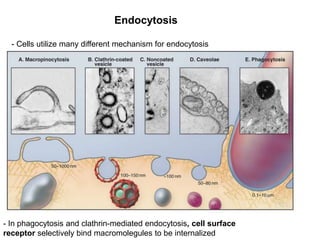
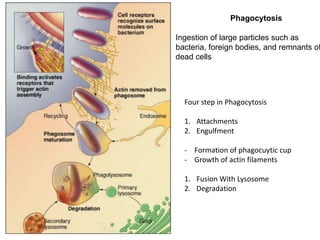
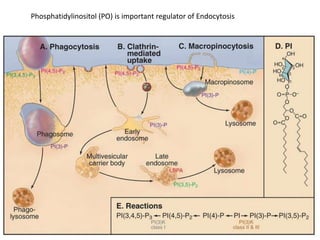
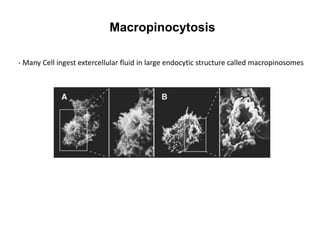
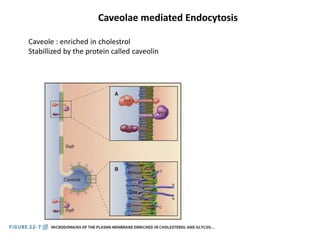
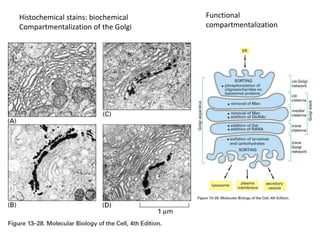
1. Membrane trafficking allows the transfer of cargo between organelles through transport vesicles that form and fuse with target membranes.
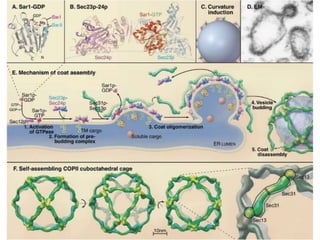
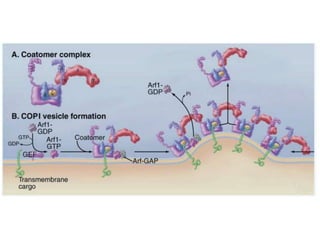
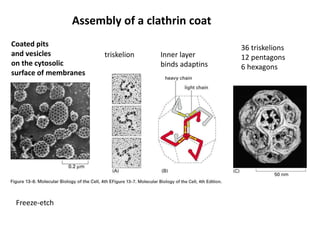
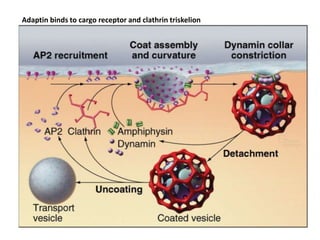
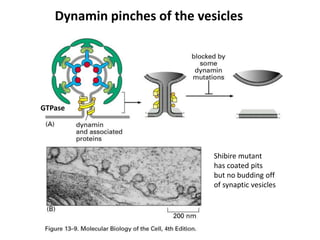
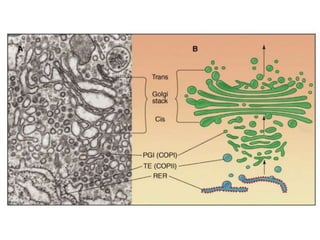
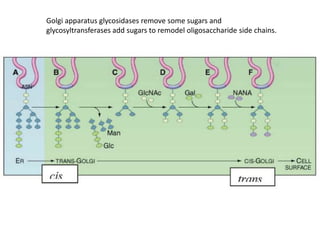
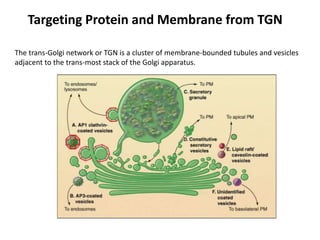
2. Transport vesicles are coated with protein complexes that help generate the vesicles and select cargo for transport. Vesicles move cargo between organelles like the ER, Golgi apparatus, and endosomes.
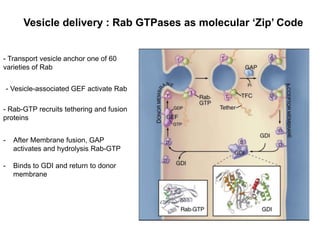
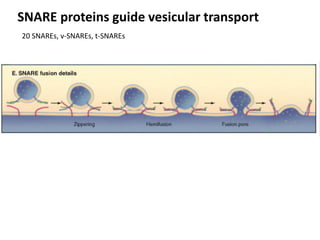
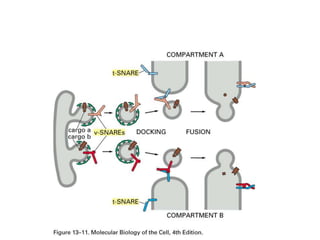
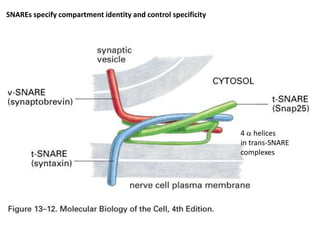
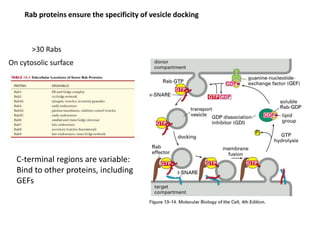
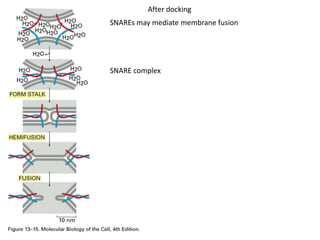
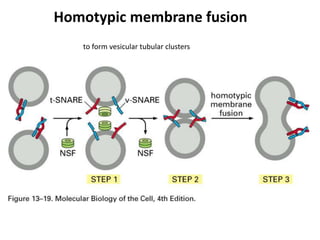
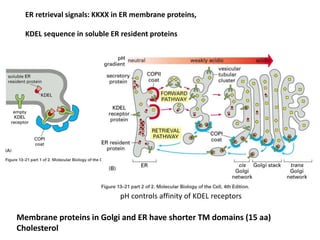
3. Rab GTPases and SNARE proteins ensure vesicles dock and fuse with the correct target membrane, delivering cargo to its destination compartment.