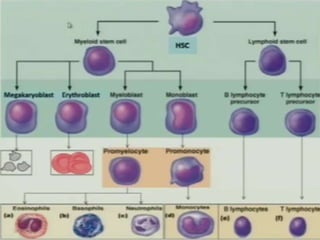
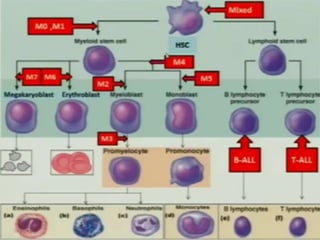
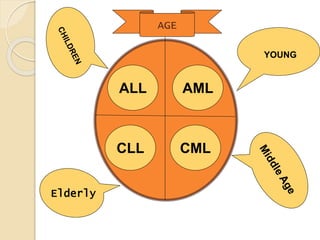
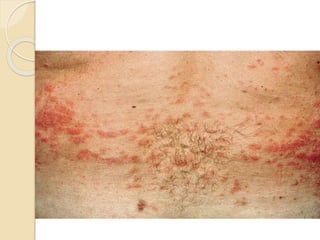
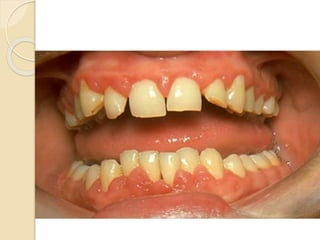
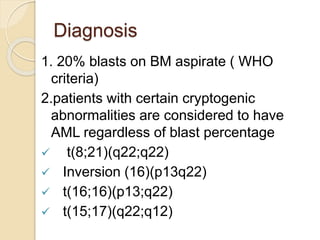
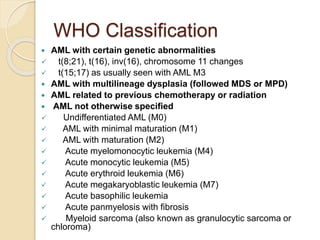
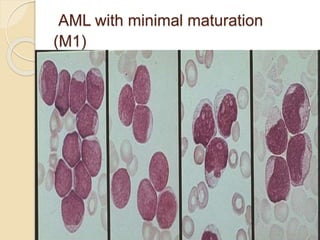
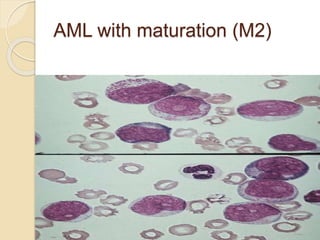
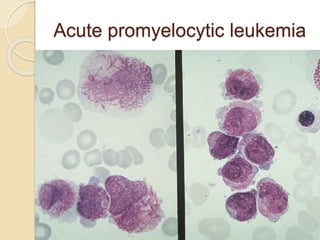
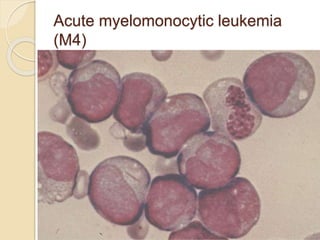
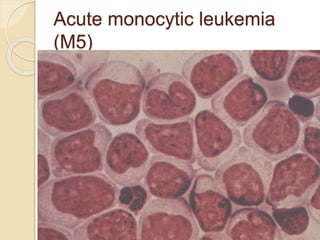
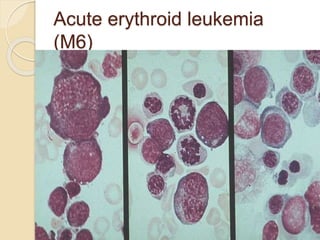
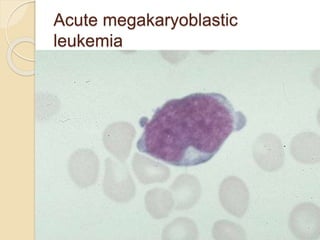
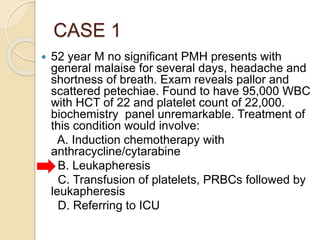
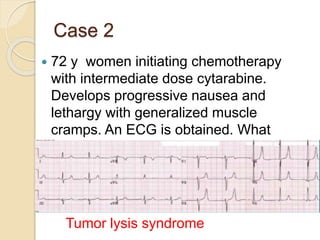
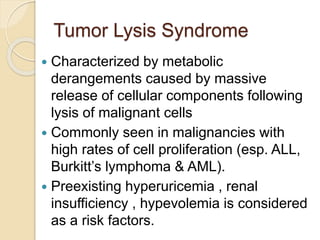
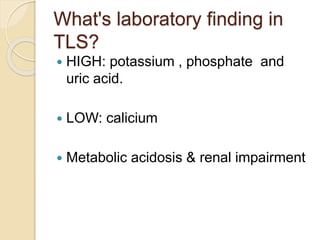
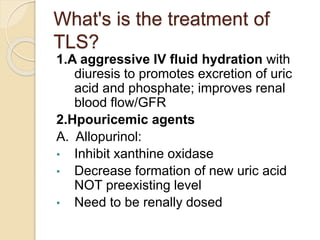
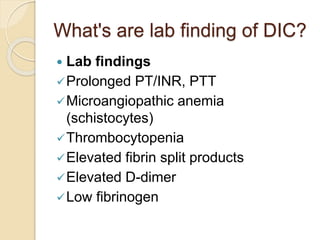
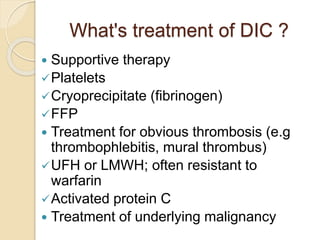
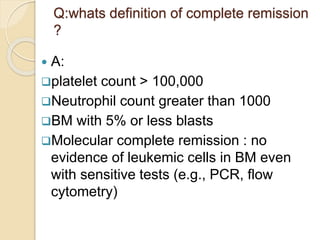
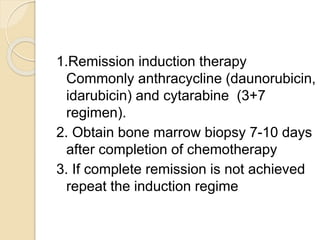
AML is characterized by accumulation of abnormal blast cells in the bone marrow and impaired production of normal blood cells. It results from clonal expansion of myeloid precursor cells with reduced ability to differentiate. Treatment involves induction chemotherapy with anthracyclines and cytarabine to achieve complete remission, defined as less than 5% blasts in the bone marrow. Risk is then assessed based on genetics to determine if additional chemotherapy or stem cell transplant is needed.