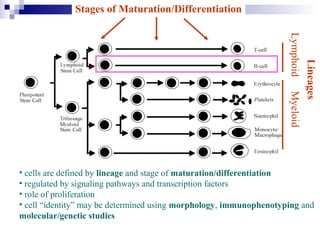
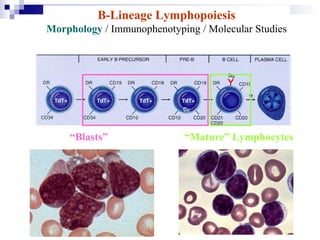
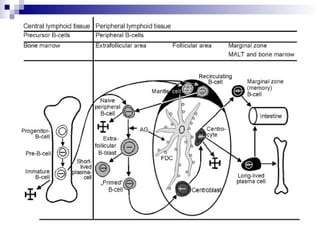
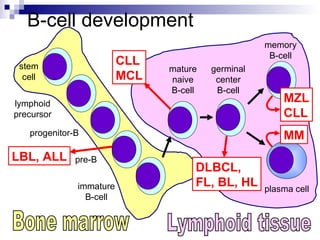
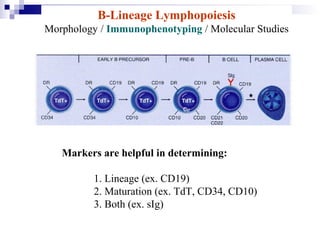
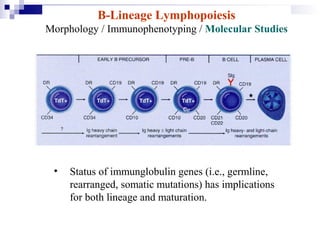
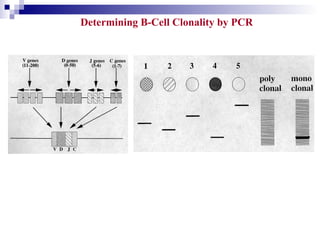
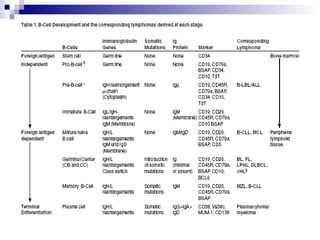
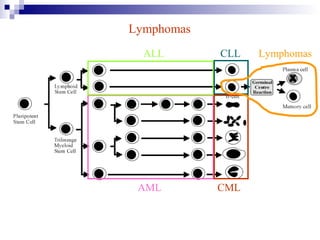
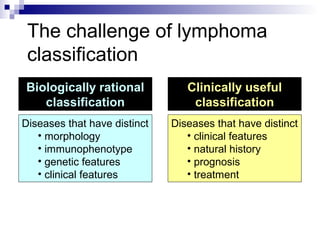
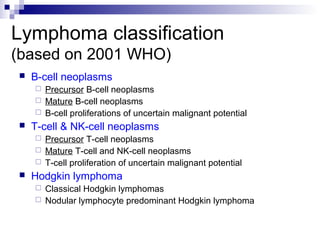
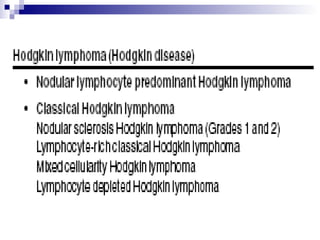
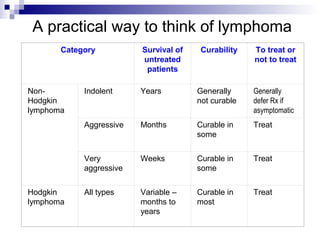
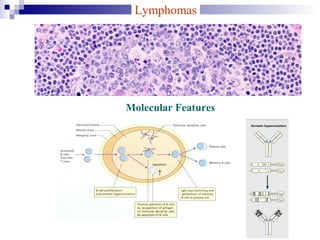
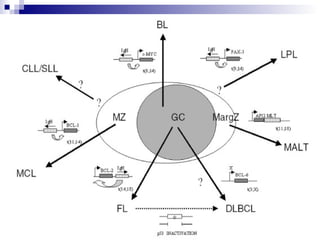
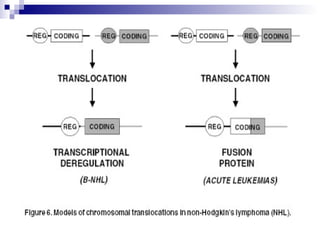
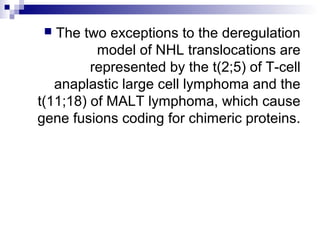
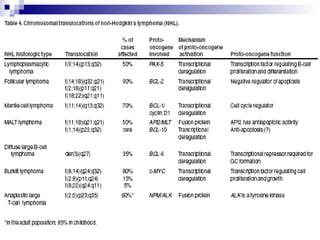
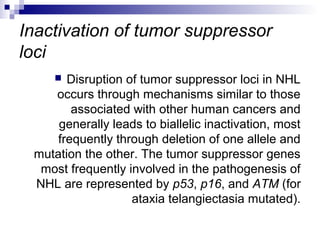
This document discusses mature lymphoproliferative disorders. It covers their classification, stages of maturation, B-cell development and lymphomagenesis. Molecular features of lymphomas include genetic alterations, infection, antigen stimulation and immunosuppression. Chromosomal translocations can activate proto-oncogenes by juxtaposing regulatory sequences. Tumor suppressor genes are also inactivated through deletion and mutation. Somatic hypermutation may introduce genetic changes involved in lymphomagenesis.