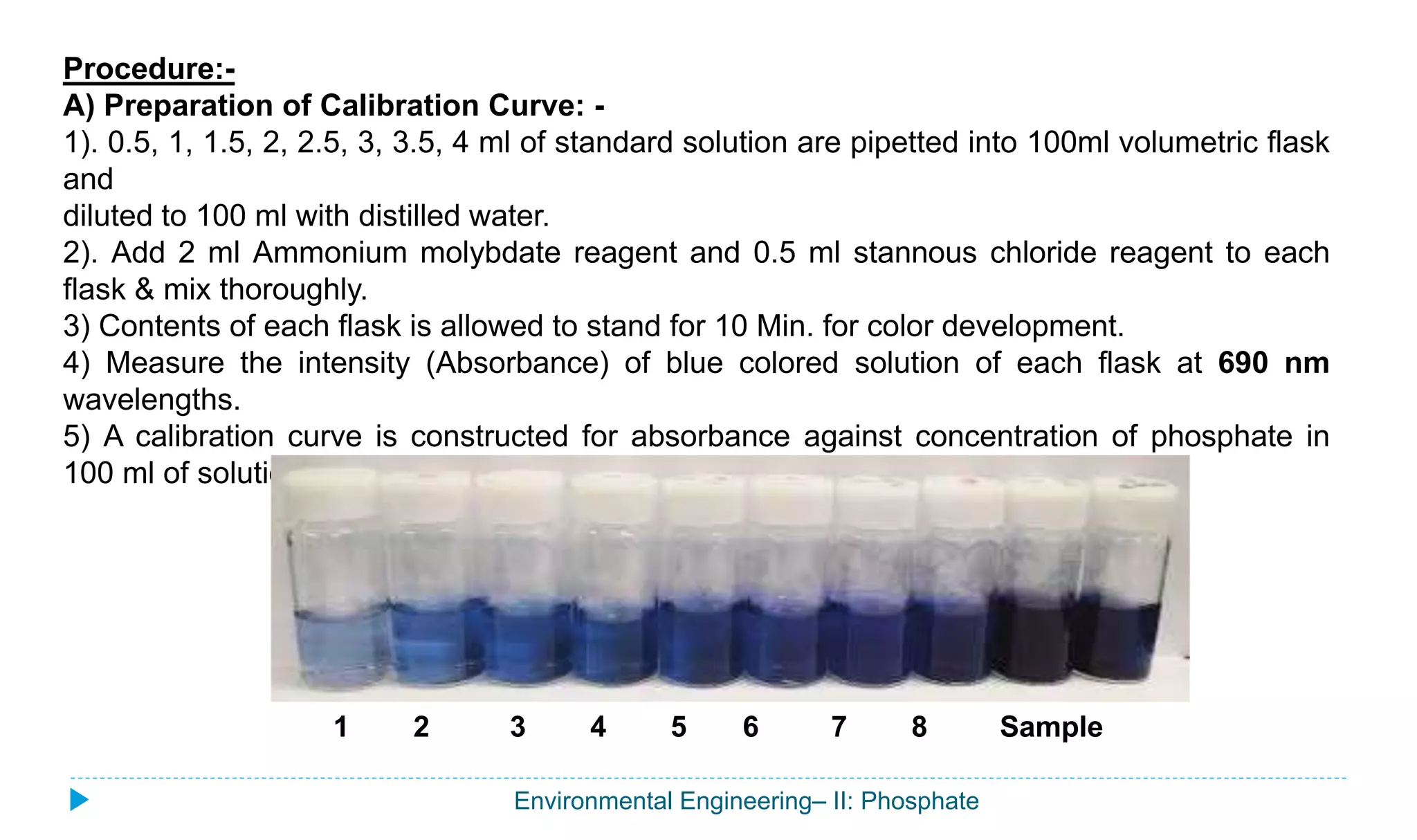
This document outlines a procedure to determine the total phosphate content of a water sample. Phosphorus plays an important role in biochemical processes and eutrophication of surface water. The main sources of phosphorus in wastewater are human excreta, household detergents, and some industrial effluents. The procedure involves preparing a calibration curve using standard phosphate solutions, then measuring the absorbance of the water sample reacted with ammonium molybdate and stannous chloride reagents to determine its phosphate concentration based on the calibration curve. The total phosphate content is calculated based on the volume of the water sample. The results will help assess eutrophication levels in surface waters affected by wastewater discharges.