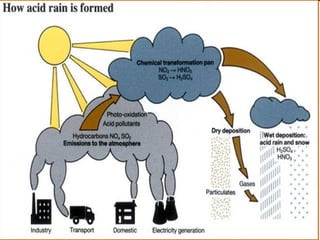
Acid rain is rain that is more acidic than normal due to sulfur dioxide and nitrogen oxides from the burning of fossil fuels and other sources. These gases mix with water vapor and rain to produce solutions of sulfuric and nitric acids that fall as acid rain. Acid rain damages buildings, statues, and other structures made of limestone and marble. It also harms forests and aquatic life by leaching nutrients from soil and increasing the acidity of lakes and rivers. To reduce acid rain, factories can install scrubbers to remove sulfur from smokestacks and vehicles can use converters to reduce dangerous emissions, while governments increase spending on pollution control and energy research.