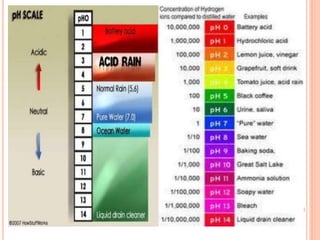
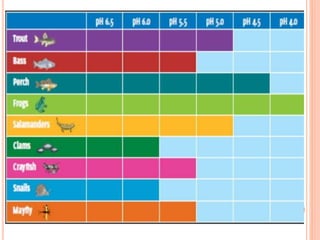
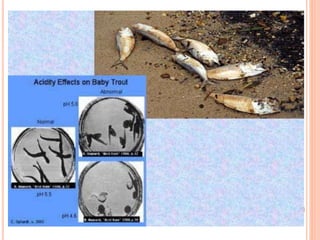
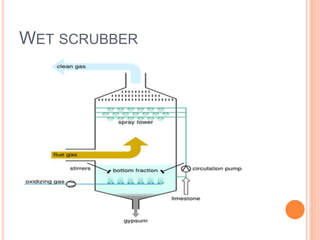
Acid rain is caused by emissions of sulfur dioxide and nitrogen oxides reacting with water in the atmosphere. It can harm plants, aquatic animals, and infrastructure by having a low pH. It impacts the environment through loss of fish in sensitive lakes and streams, eliminating insect life and fish species. Prevention methods include vehicle emission controls, fluidized bed combustion in power plants, and wet scrubbers that inject limestone to remove sulfur dioxide from smokestack gases.