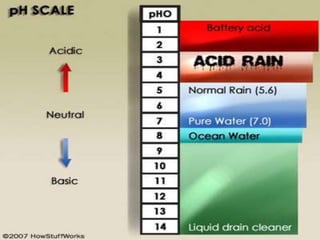
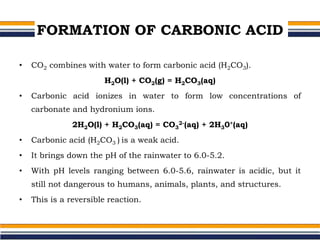
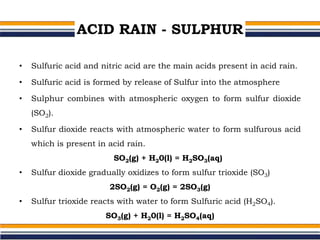
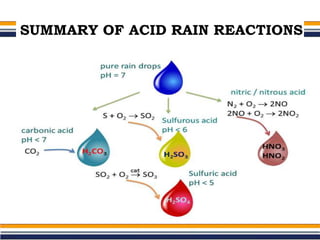
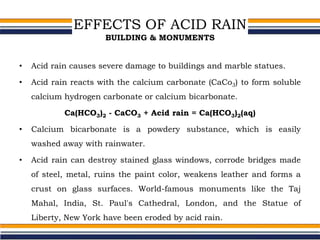
The document provides an overview of acid rain, discussing its formation from the combination of rainwater, carbon dioxide, and various gaseous pollutants like sulfur and nitrogen oxides. It outlines the effects of acid rain on buildings, plants, soils, water bodies, and human health, highlighting the damaging impact on ecosystems and architecture. Solutions to combat acid rain include reducing fossil fuel use and transitioning to alternative energy sources.