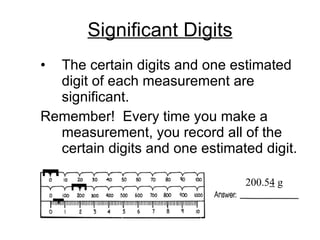
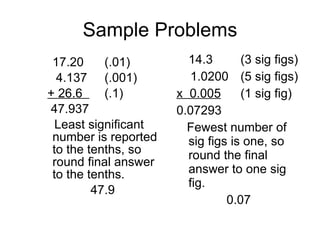
Scientific notation is a way of writing numbers as a product of two numbers: a coefficient and a power of 10. It is used to express very large and very small numbers in a way that is easier to work with. To write a number in scientific notation, the significant digits are rewritten as a number between 1 and 10, and the number of places the decimal is moved is written as the exponent. Positive exponents indicate places moved to the right, and negative exponents indicate places moved to the left. Significant figures, or sig figs, refer to the certain and estimated digits in a measurement. Rules for sig figs determine how calculations are carried out and final answers rounded.