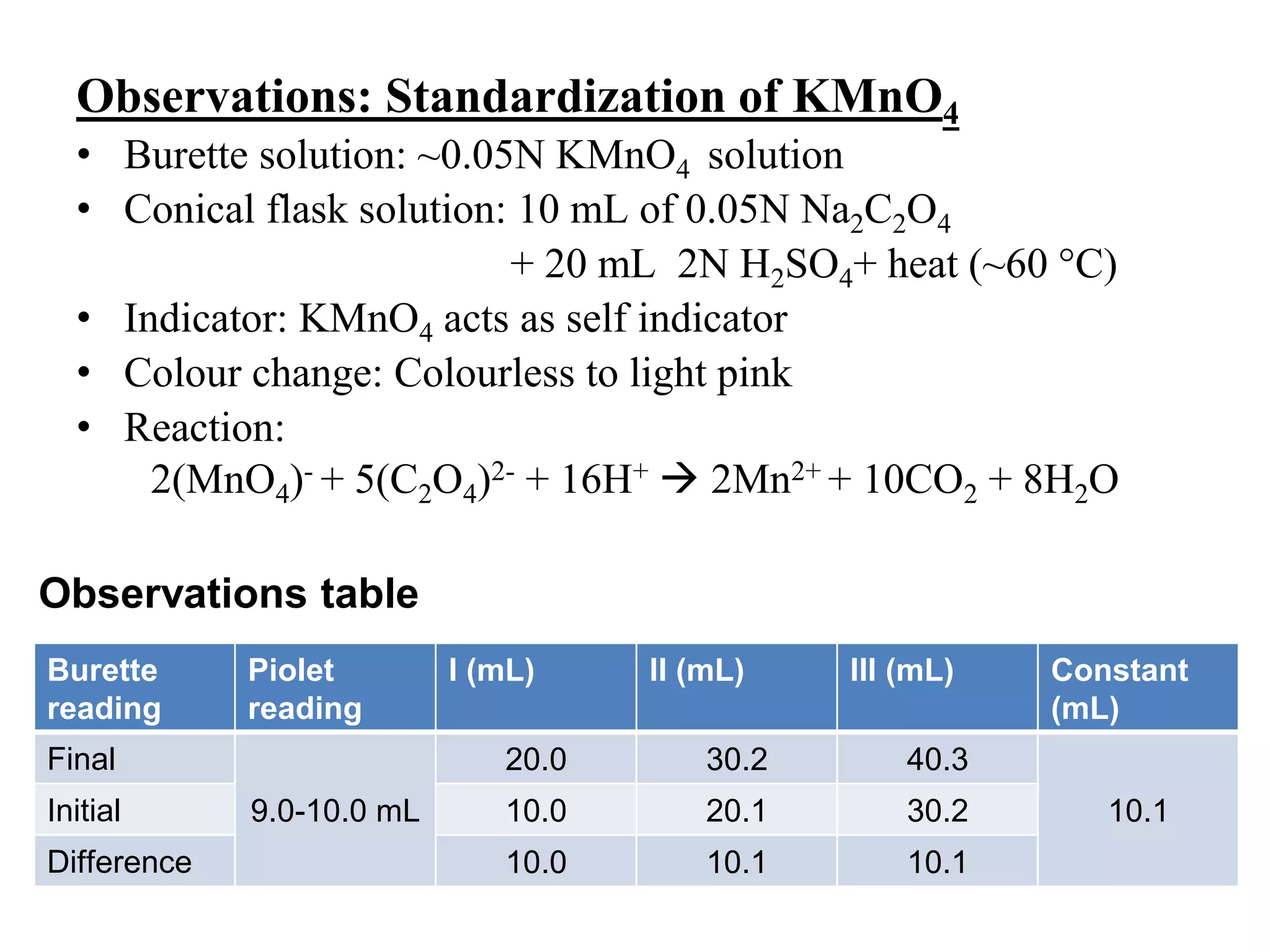
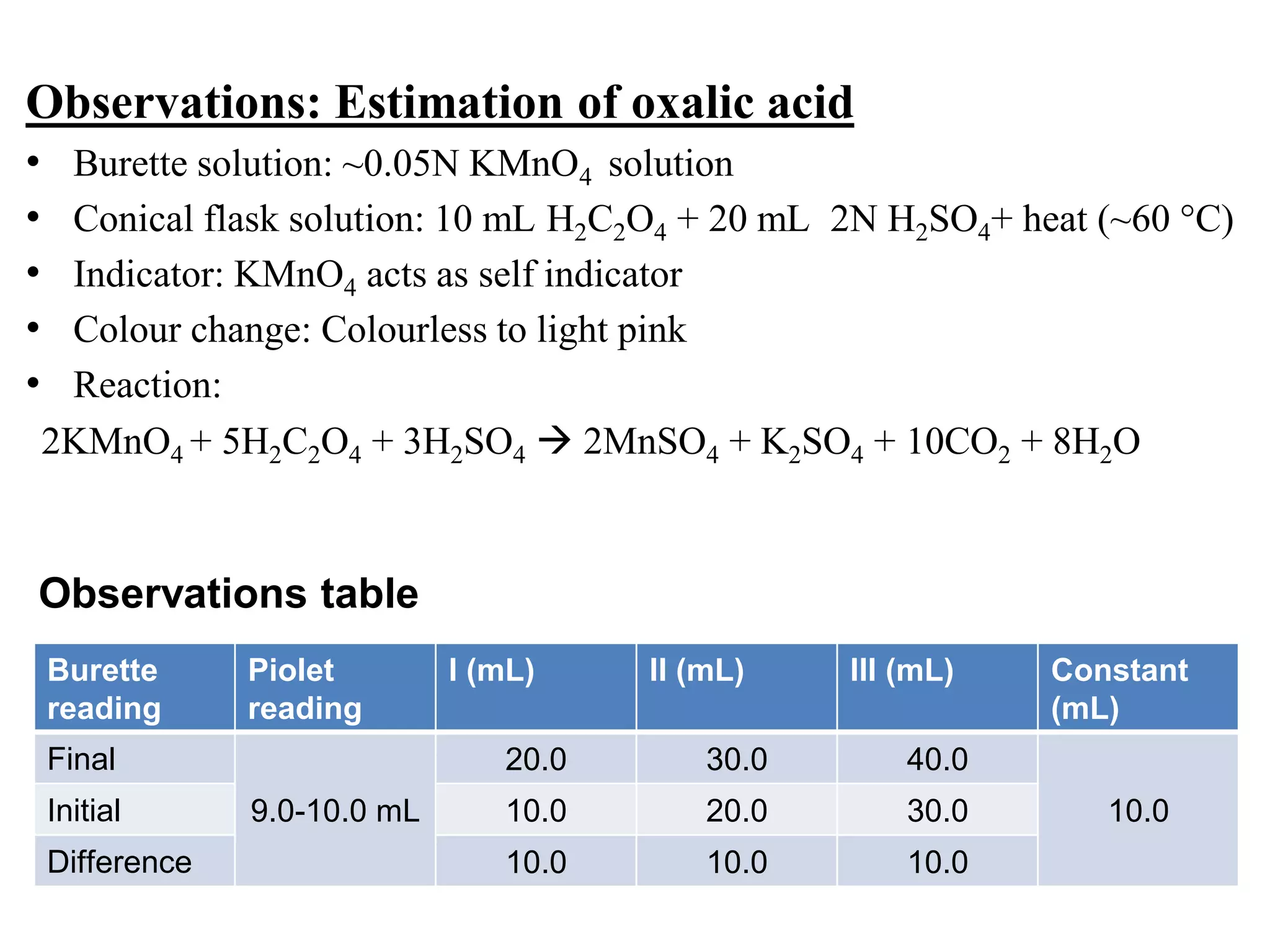
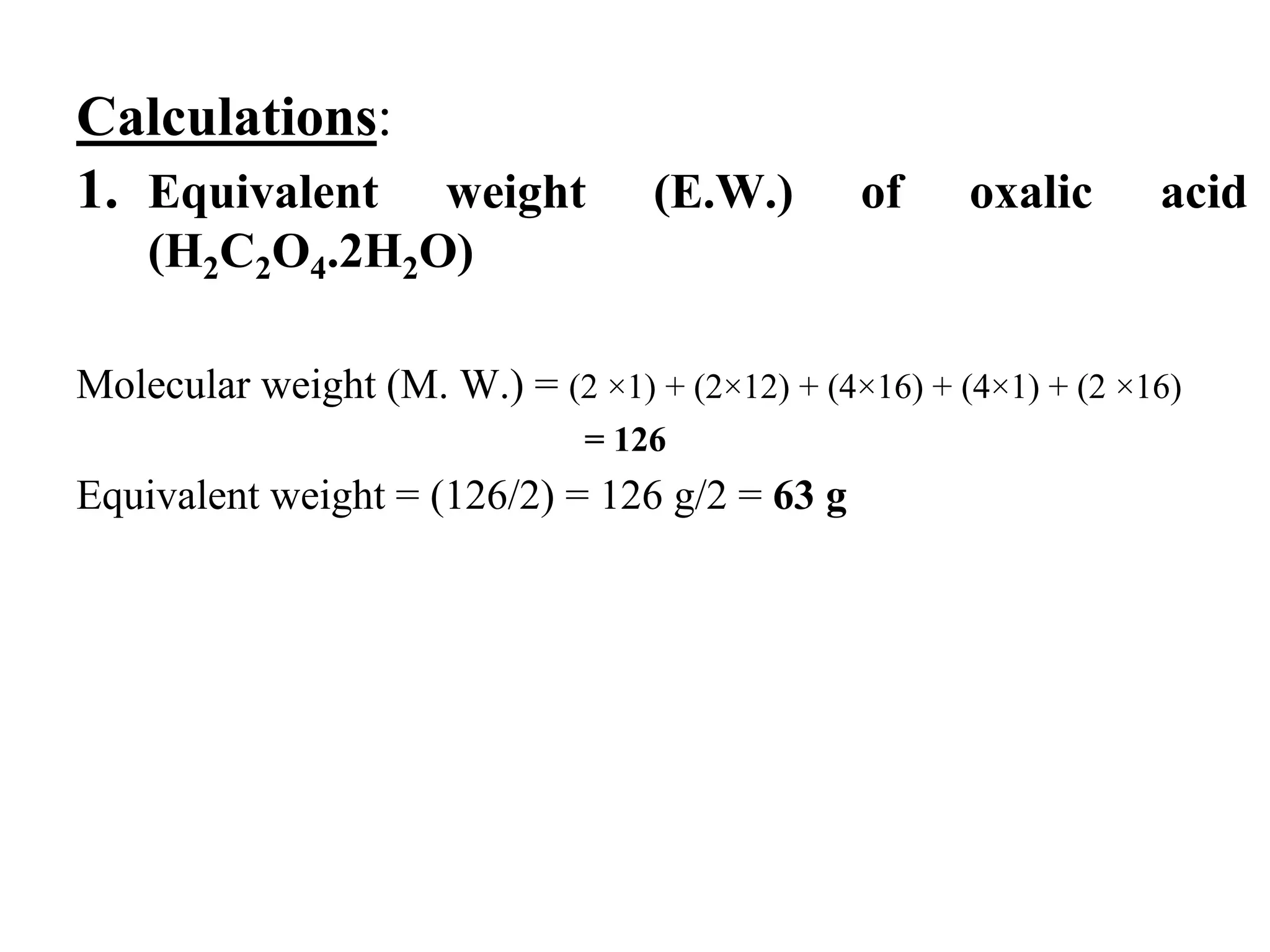
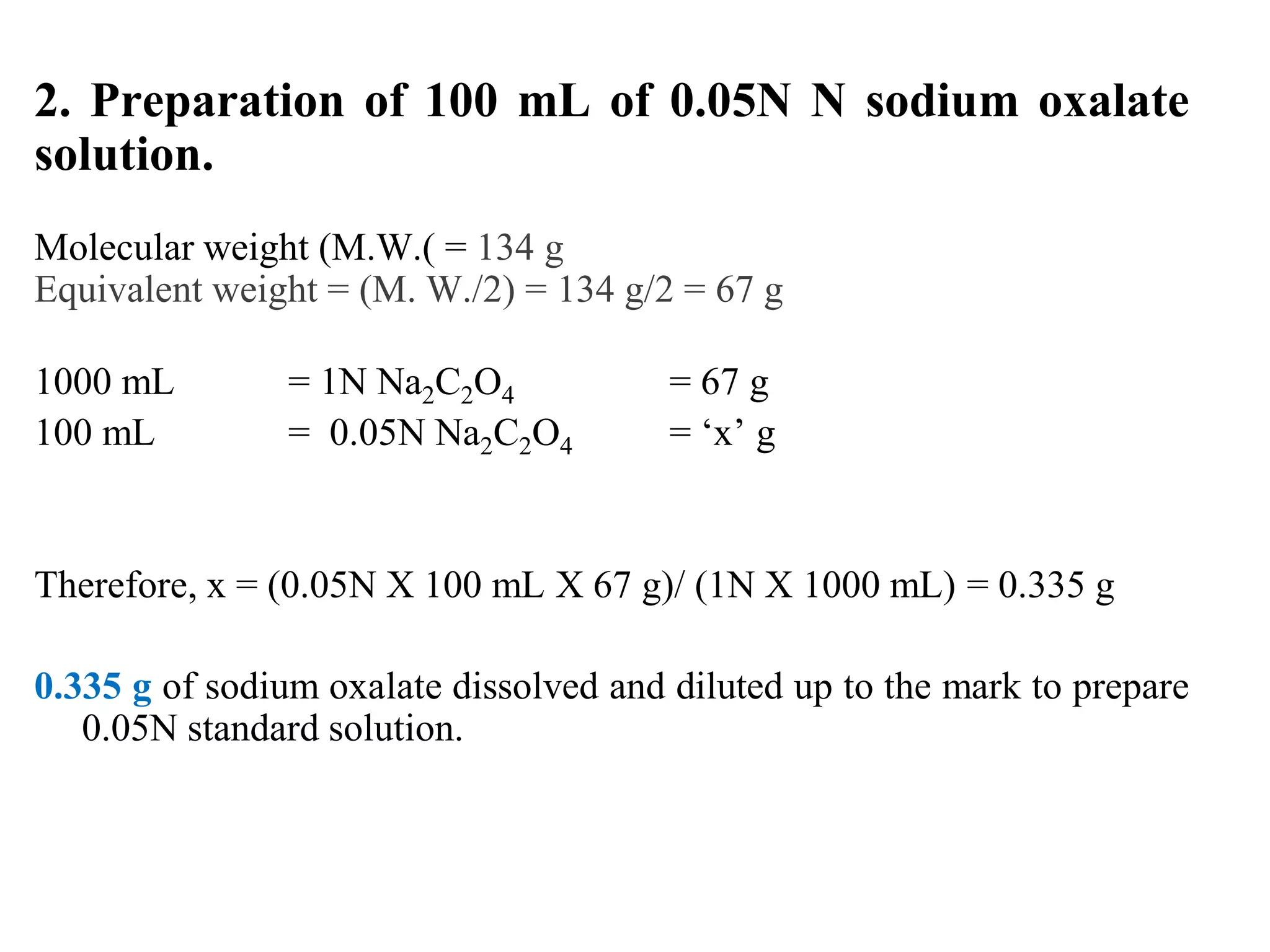
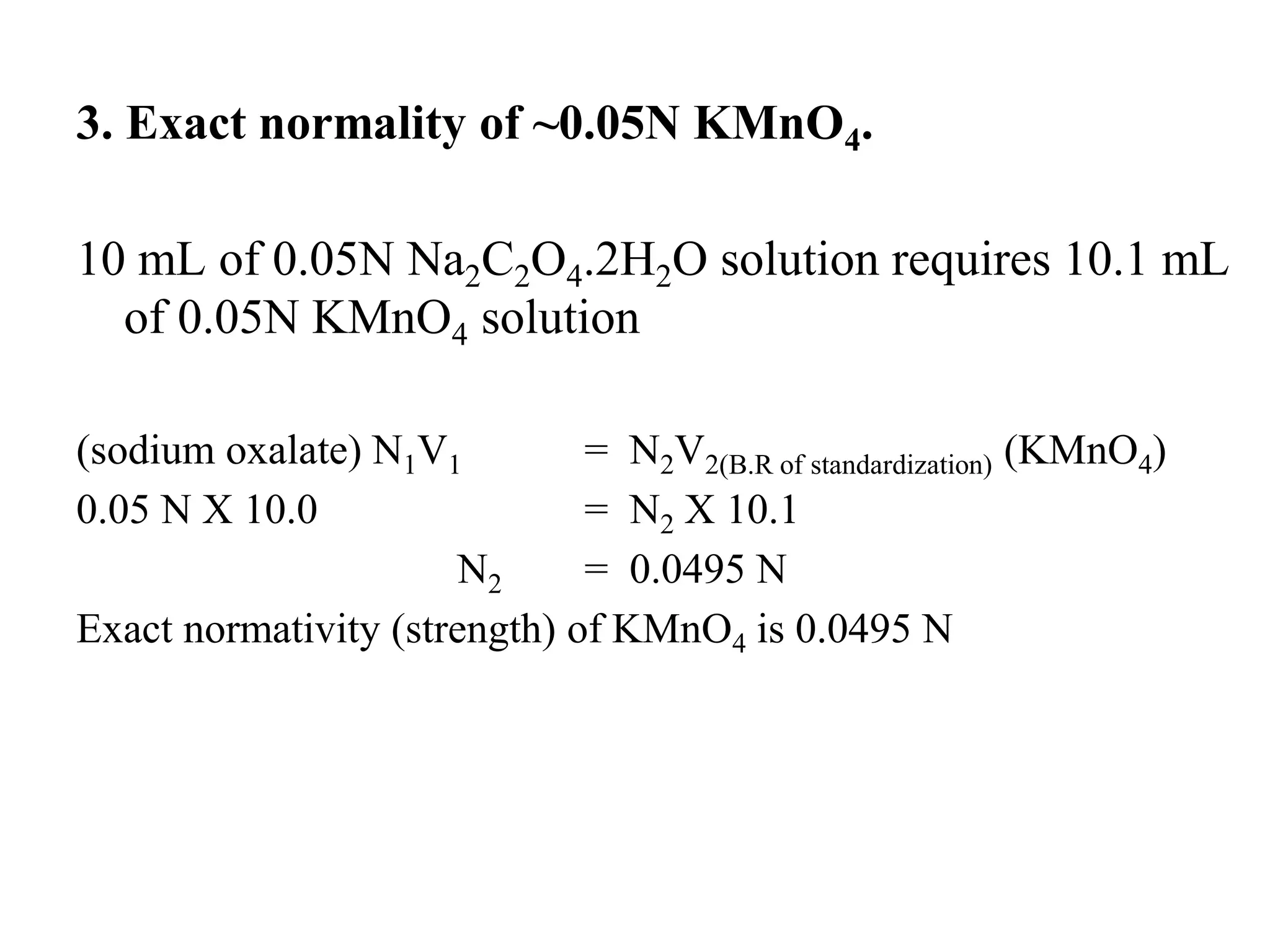
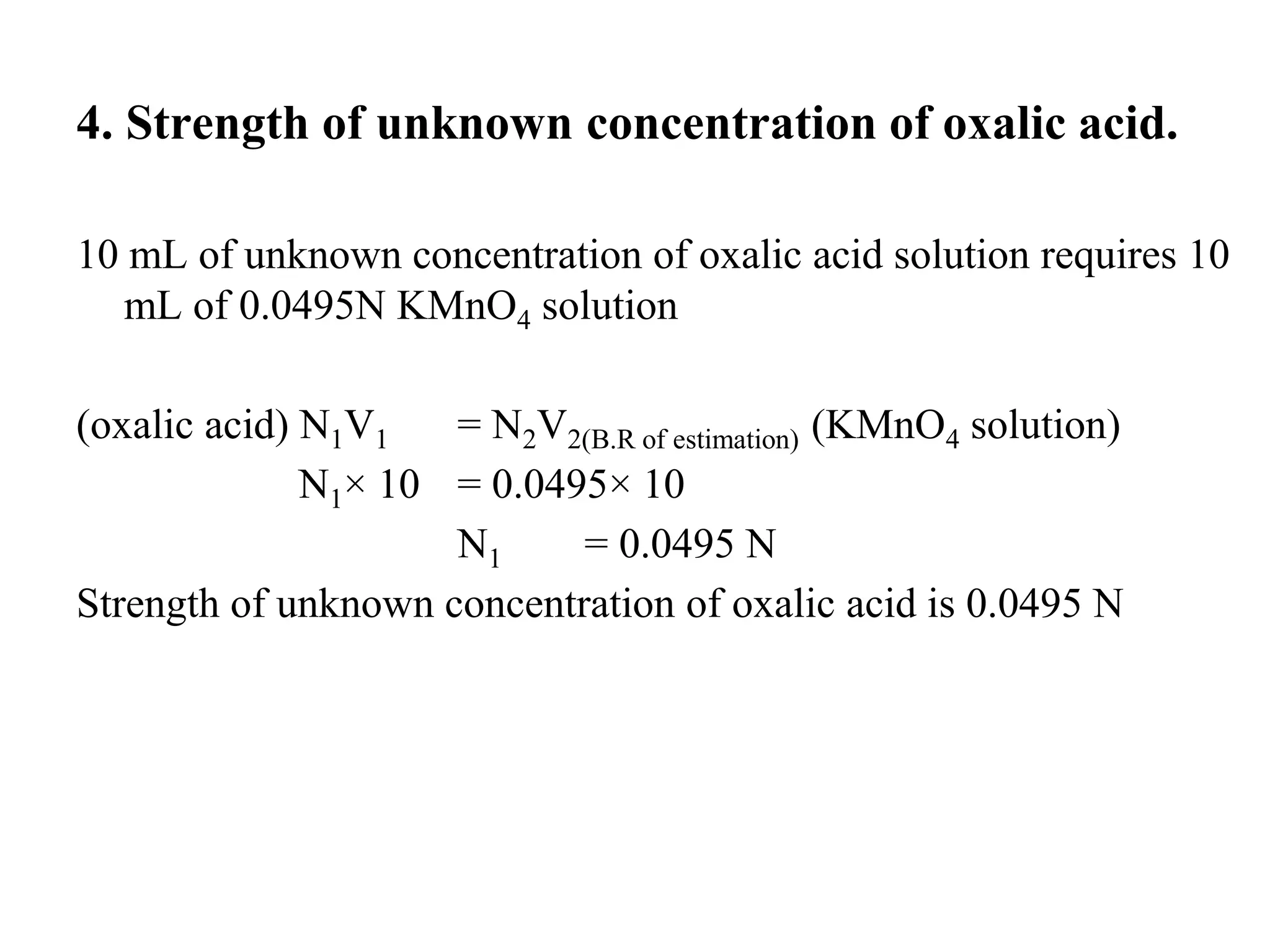
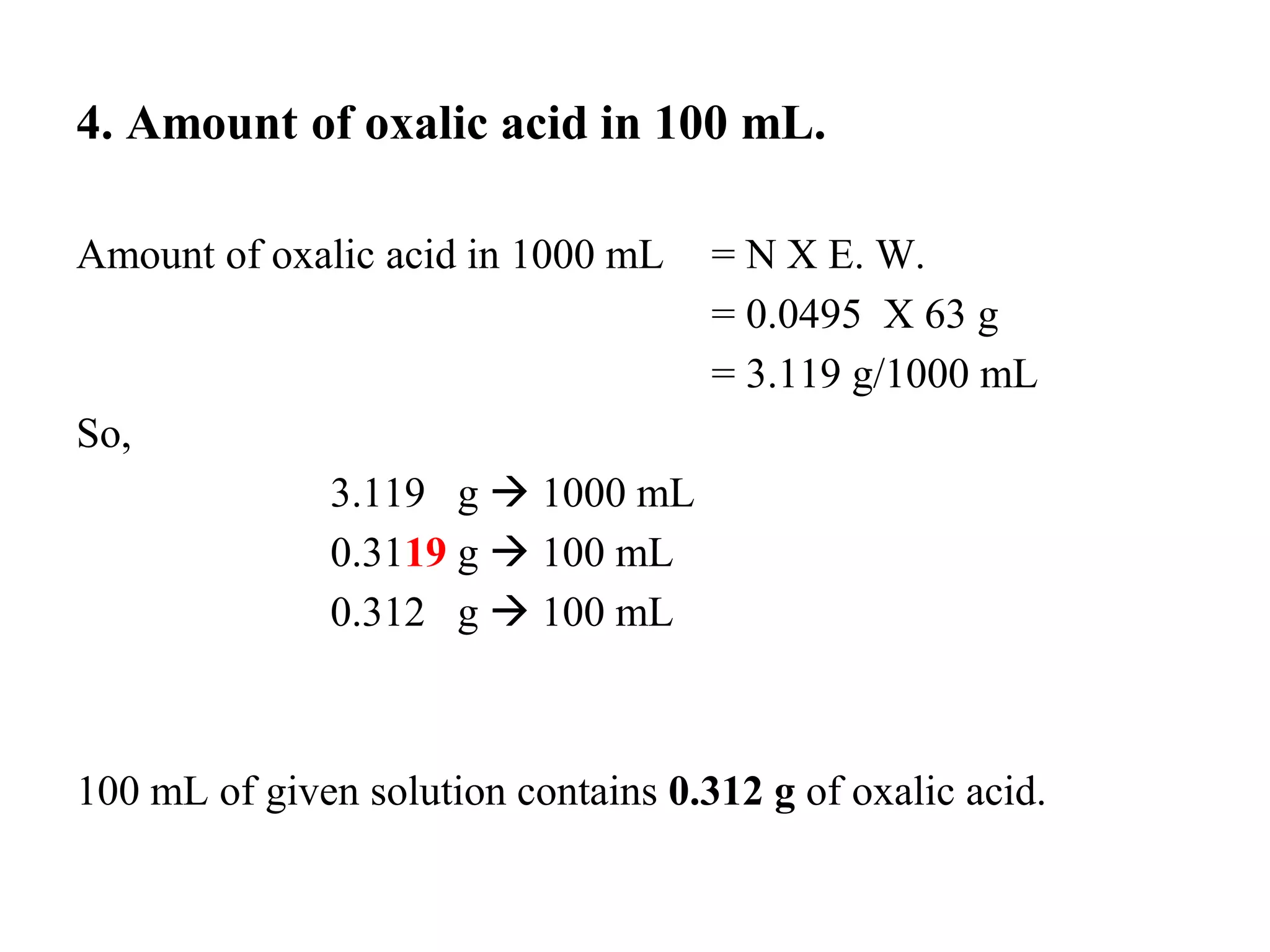
The document describes the titration of oxalic acid with potassium permanganate. It provides the theory of the redox reaction, where manganese is reduced and carbon is oxidized. It then gives the experimental procedure for standardizing the KMnO4 solution using sodium oxalate and then using the standardized solution to determine the concentration of an unknown sample of oxalic acid. The calculations section shows how to determine the equivalent weight of oxalic acid and use the titration data to find the exact normality of KMnO4 and the normality and mass of oxalic acid in the unknown sample.