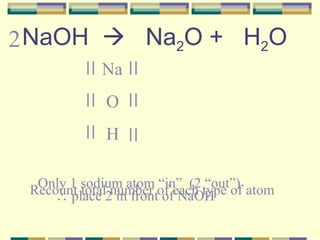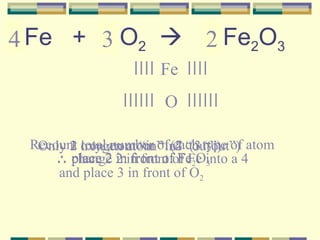This document provides an overview of chemical reactions including:
1. Key vocabulary like reactants, products, and chemical equations.
2. How to write and balance chemical equations.
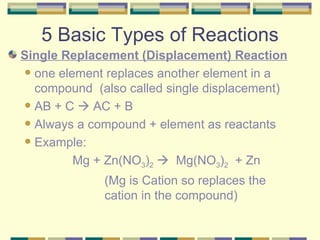
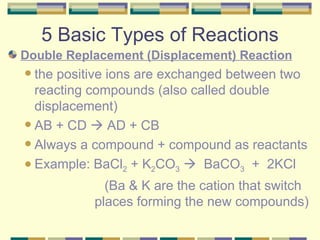
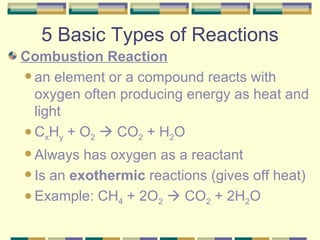
3. The five basic types of chemical reactions.
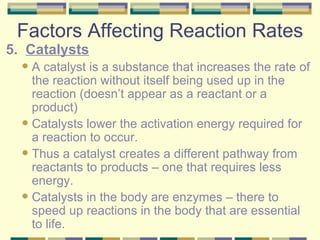
4. Factors that affect the rate of chemical reactions like temperature, concentration, and catalysts.