The document discusses acid-base balance in the human body, highlighting the importance of maintaining a stable pH through various mechanisms such as chemical blood buffers, respiratory excretion, and renal function. It covers disorders related to acid-base imbalances, including metabolic and respiratory acidosis and alkalosis, and outlines clinical evaluation methods for diagnosing these conditions. Key concepts include compensatory responses, the role of bicarbonate, and the Henderson-Hasselbalch equation in understanding acid-base homeostasis.



![Human Acid-base Homeostasis
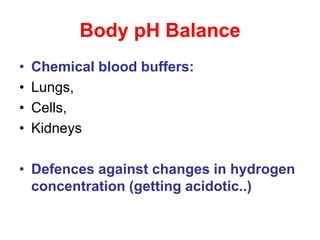
• Tight regulation:
• CO2 tension
– by respiratory excretion (of volatile acids)
• Plasma bicarbonate [HCO3
-]
– By renal HCO3
- reabsorption and
– Elimination of protons produced by
metabolism
• pH is determined by CO2 tension and
[HCO3
-]](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-6-320.jpg)
![Physiology of Buffering:
• Ability of a solution containing a weak or poorly
dissociated acid and its anion (a base) to resist
change in pH when strong acid or alkali is added
• 1 ml of 0.1 M HCl to 9 ml distilled water =
• [H+] from 10 -7 M to 10 -2 M= pH from 7 to 2
• 1 ml of 0.1 M HCl to 9 ml of phosphate buffer:
dissoc. H+ combines with [HPO4
2-] = (H2PO4
-)
• pH fall of only 0.1= to 6.9](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-7-320.jpg)
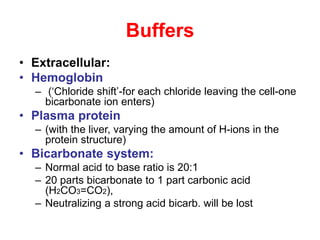
![Bicarbonate Buffer
• Extracellular most important buffer
• Proteins and phosphate buffer less
important
• Intracellular phosphate- most important b.
• Equilibrium conditions because abundant
carbonic anhydrase in blood
• H+ + HCO3
- H2CO3 H2O + CO2
• [H+ ]= Keq x [H2CO3 ]/[HCO3
-]](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-8-320.jpg)
![Equations
• H+ + HCO3
- H2CO3 H2O + CO2
• Equilibrium
• [H+ ]= Keq x [H2CO3 ]/[HCO3
-]](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-9-320.jpg)
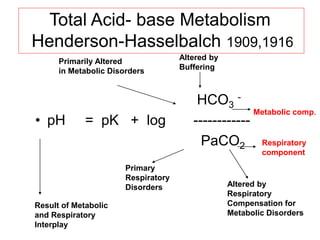
![Normal Values
• [HCO3
-] ~ 24 mM
• PaCO2 = 38 torr
• pH ~ 7.42
• Plasma HCO3
- regulation by
– reclaiming filtered HCO3
- and
– generating new HCO3
- (carboanhydrase)
– ( to replace the lost internally titrating metabolic acid
and externally from the GI tract)
• Production of 1 mmol of acid/kg body weight
per day (60 kg=60 mmol/day)](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-11-320.jpg)
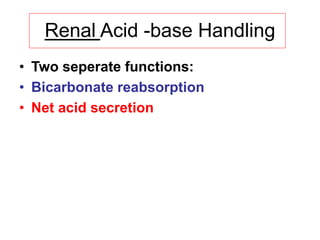
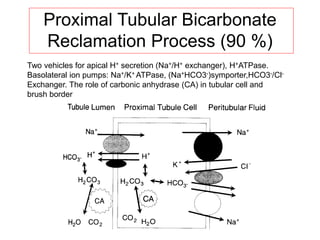
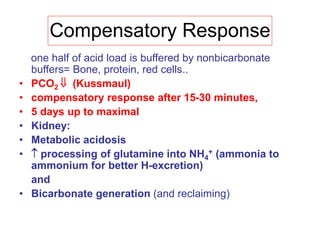
![Net Acid Excretion
• Urine is acid = pH~ 4.5
• Buffer salts are in the tubular fluid
• Phosphate is the most important buffer in urine:
HPO4
2- + H+ = H2PO4
-
• Nonvolatile (fixed) acids (anions= sulfates,
phosphates) must be accompanied in the urine
by equivalent cations (Na +, K +, Ca + +) for
maintenance of electrical neutrality
• In acid urine ammonium helps to keep
[H+ ] (ammonia NH3
+ to NH4
+)](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-14-320.jpg)
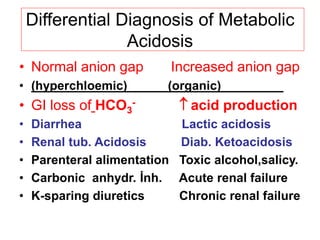
![Metabolic acidosis
• Hallmark is [HCO3
-]
• 1. Acid production net acid intake
above net renal excretion
(ketoacidosis, lactic acidosis, ammonium
chloride loading)
• 2. failure of renal net excretion
(chronic renal failure, renal tubular acidosis)
• 3. Bicarbonate loss via the gastroinestinal
tract (diarrhea, gastrointestinal fistula)
• 4. Nonbicarbonate solutions added to ECF
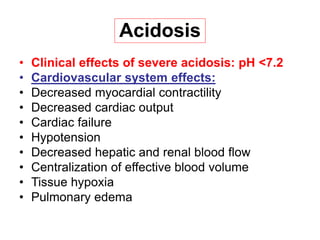
(dilutional acidosis)](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-17-320.jpg)
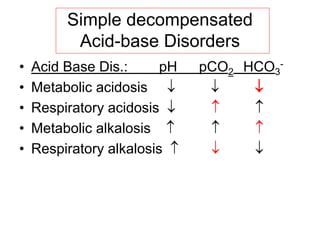
![Steps of evaluation
• 1. Examine pH= Reduction ( 7.2) Acidosis
• Increase (7.5) Alkalosis
• 2. Examine directional change of PCO2
• and [HCO3
-] ,
• pH acid, HCO3
- low Metabolic acidosis
• pH alkal., HCO3
- high Metabolic alkalosis
• 3. Assess degree of compensation: Mixed acid-base
disorder?
• Metabolic acidosis PCO2
• Metabolic alkalosis PCO2
• Failure of respiratory compenstion= primary
respiratory acid-base disorder
• Never to initial pH through compensation !!](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-18-320.jpg)
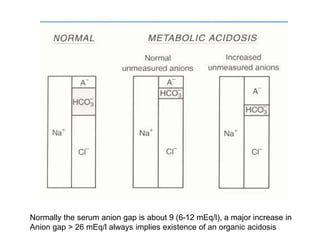
![• 4. Calculate the serum anion gap
• Is the acid-base disorder organic or
mineral in origin??
• We use venous sample blood electrolytes:
• Electroneutrality demands:
• Serum anion gap, that means:
• [Na+] + [UC]= [Cl-] +[Total CO2] + [UA]
• (U means: unmeasured)
Steps of evaluation](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-19-320.jpg)


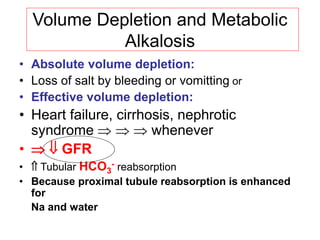
![Metabolic Alkalosis
• Plasma bicarbonate [HCO3
-] = pH
• 1) H+ GI loss or shift into cells
• 2) Excess HCO3
-
Administration of bicarbonate, or precursors:
lactate, acetate, citrate or
Failure to excrete: mineralocorticoid effect
• 3) Loss of fluid with
Diuretic therapy
[Cl-], [K+] and [H+] loss from plasma-
extracellular volume contraction](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-27-320.jpg)




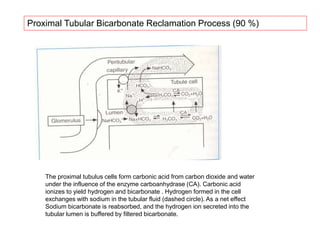
![Henderson-Hasselbach 1909,1916
• H2CO3 = p CO2 + solubility in physiol.
Fluids
• [H+ ]= K x [S x pCO2 ]/[HCO3
-]
Antilog of both sides:
pH= pK + log10 [HCO3
-] / [S x PCO2]
In blood at 37º C, pK =6.1 and S is 0.03
pH= 6.1+ log10 [HCO3
-] / [0.03 x PaCO2]](https://image.slidesharecdn.com/acid-basebalanceanditsdisorders-240504174843-0af5bb2d/85/Acid-base-Balance-and-its-Disorders-presentation-37-320.jpg)