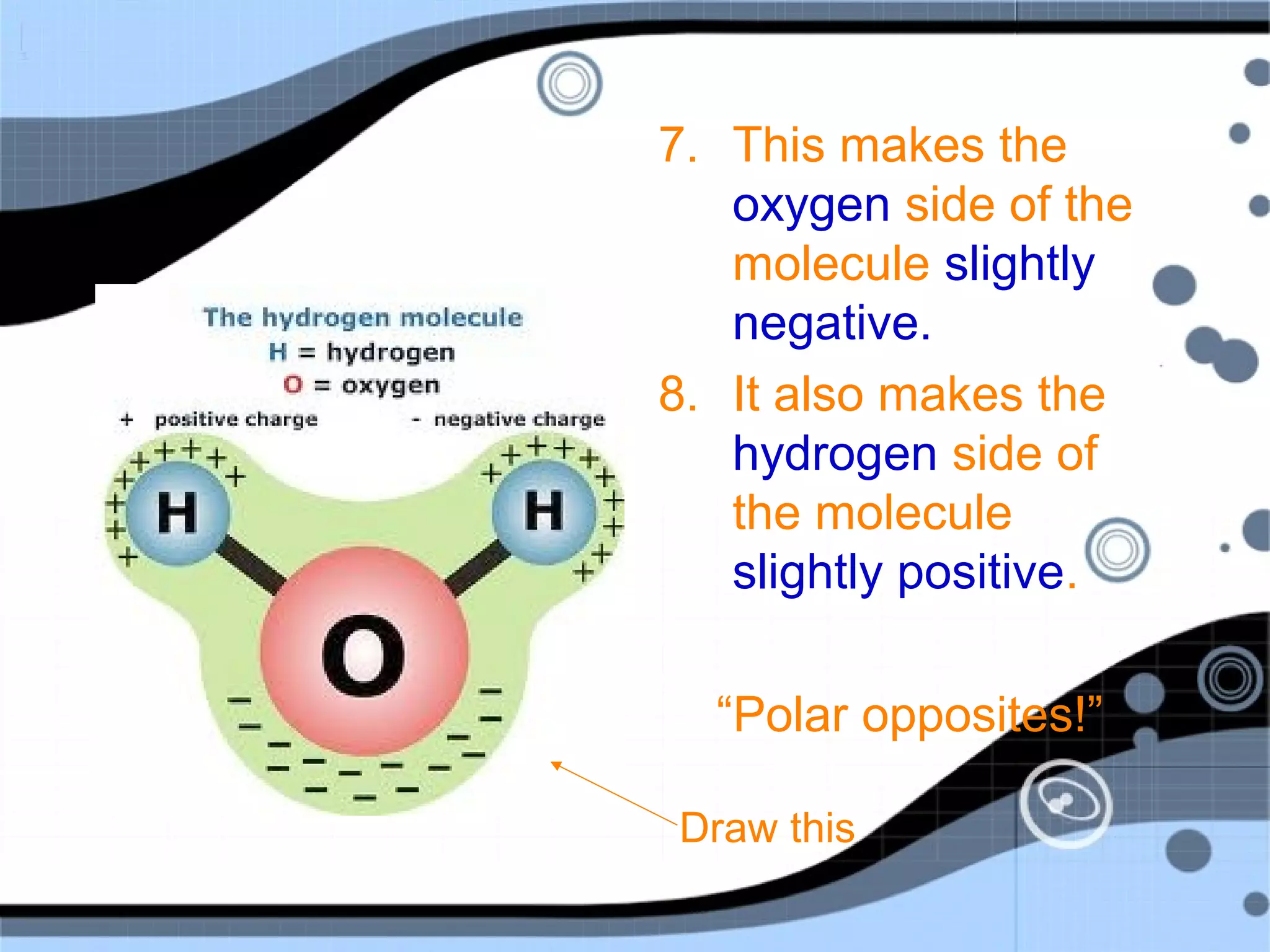
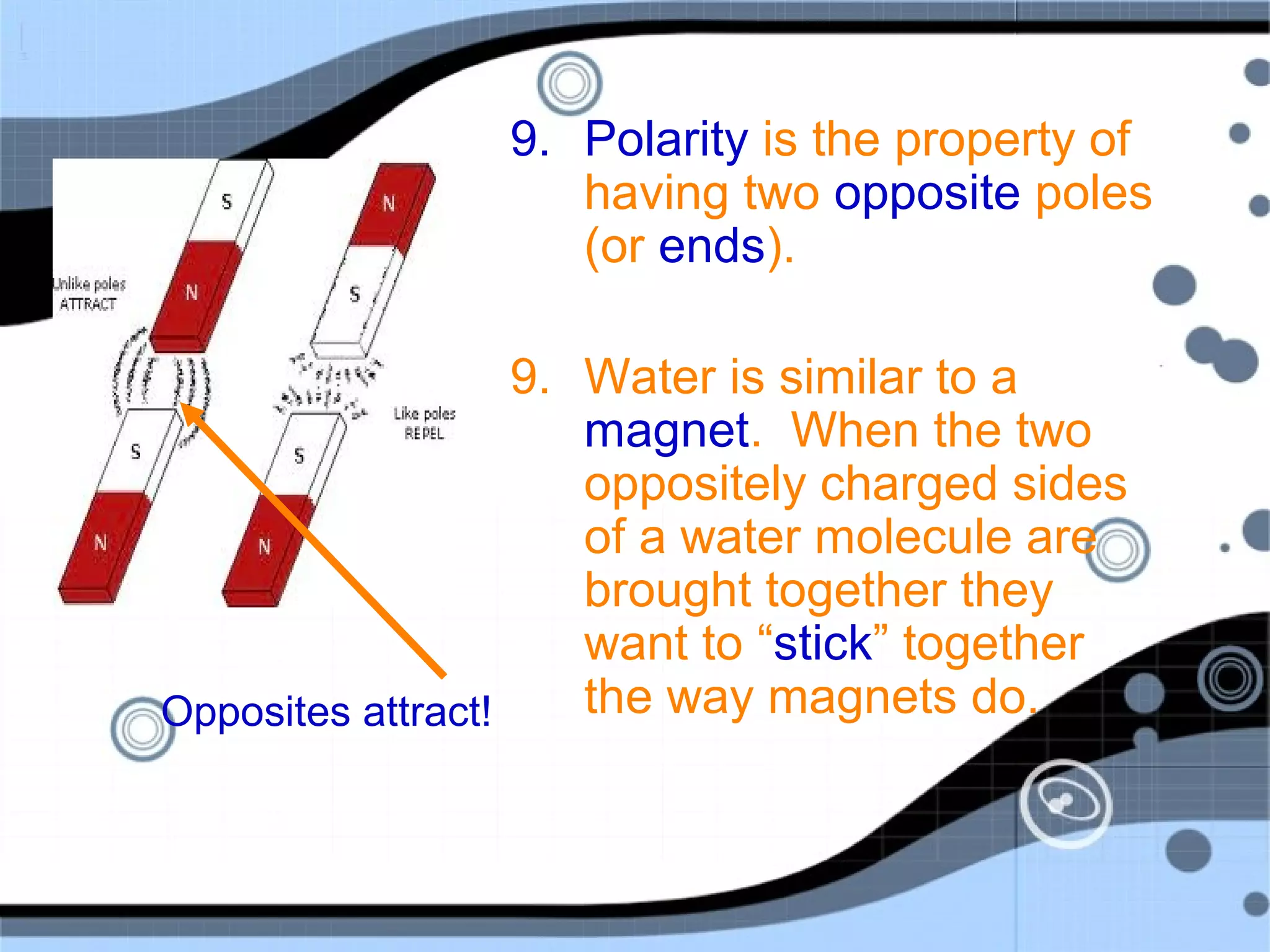
1. Water is a polar molecule made up of oxygen and hydrogen atoms, with the oxygen side having a slight negative charge and the hydrogen sides having a slight positive charge.
2. This polarity allows water molecules to form hydrogen bonds with other water molecules, giving water its unique properties. Water is able to dissolve many other polar substances for this reason.
3. The hydrogen bonding between water molecules also gives water properties like high surface tension and the ability to exist as a liquid at temperatures higher than zero degrees Celsius. This hydrogen bonding is important for life.