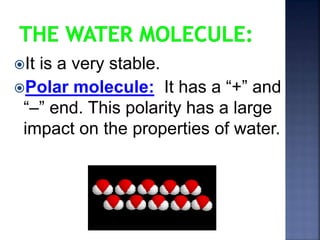
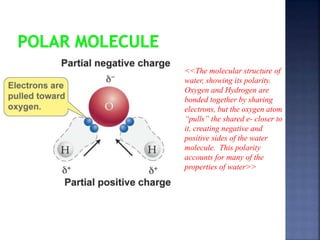
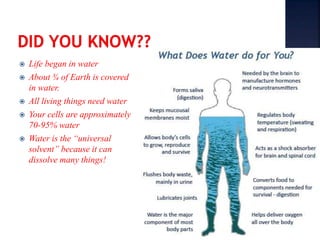
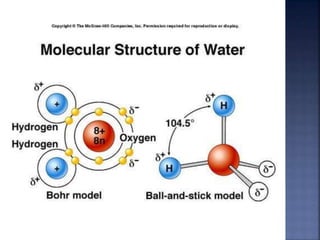
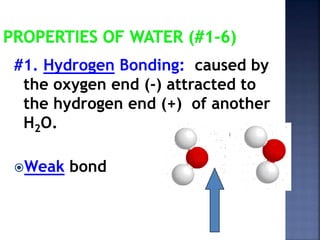
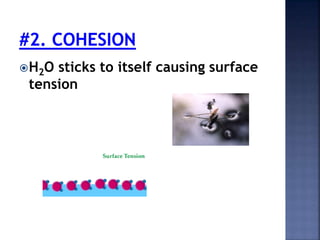
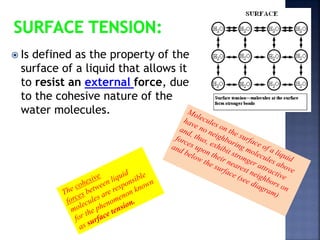
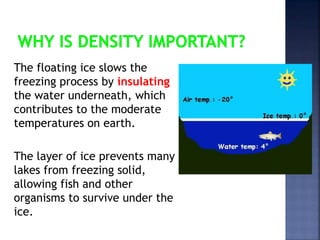
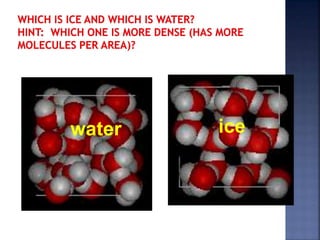
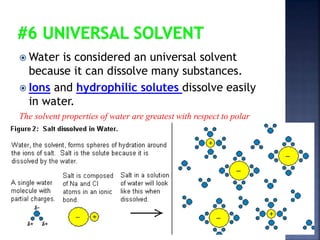
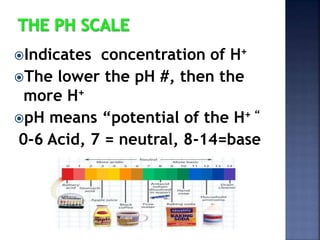
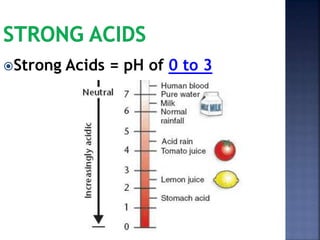
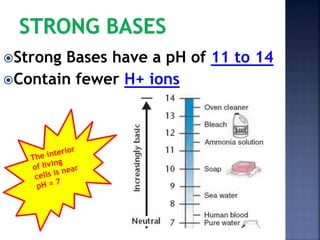
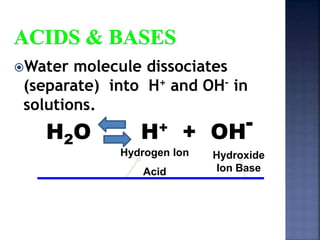
The document discusses the molecular structure and properties of water. It notes that water is a polar molecule, with slightly positive and negative ends due to the uneven sharing of electrons between the oxygen and hydrogen atoms. This polarity allows water molecules to form hydrogen bonds with each other and other polar substances. The hydrogen bonding between water molecules gives water many unique properties that help to moderate Earth's temperature and make it suitable for life, including its high heat capacity, ability to change state from solid to liquid to gas, and ability to dissolve many other substances.