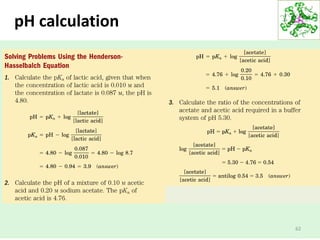
This document provides an overview of the key topics covered in a lecture on the biochemistry of water, including:
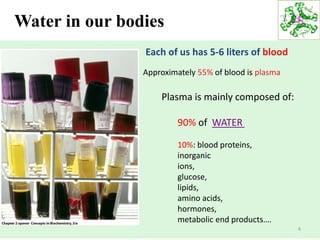
- Water makes up a large percentage of living organisms and is essential for life.
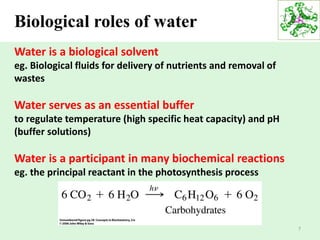
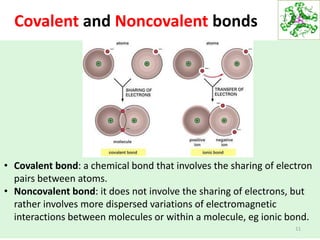
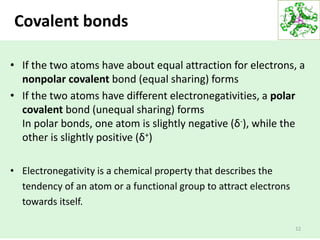
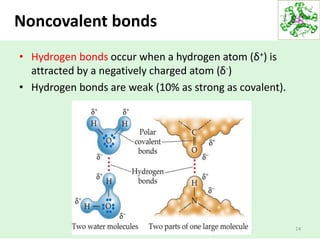
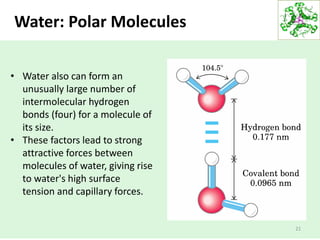
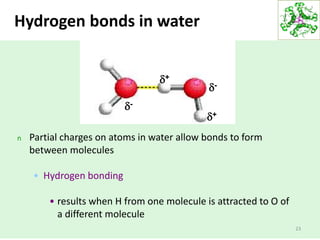
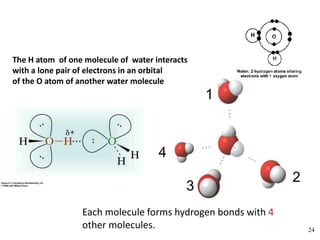
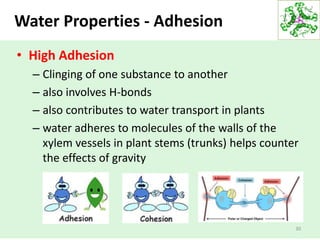
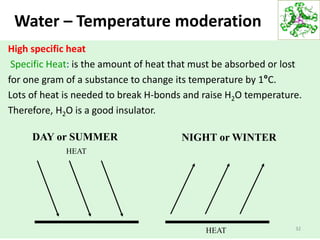
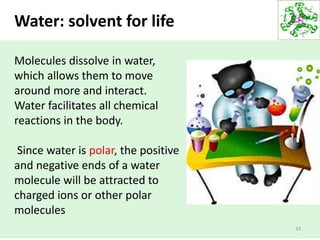
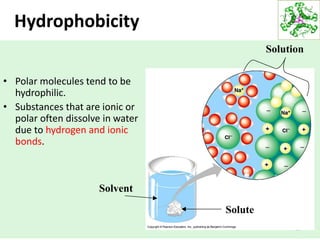
- Water's unique hydrogen bonding properties give it high cohesion, adhesion, surface tension, and specific heat capacity, allowing it to act as a solvent and moderate temperatures in biological systems.
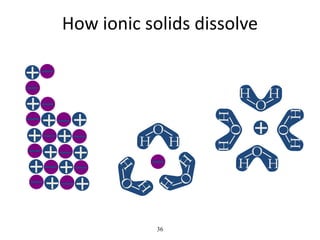
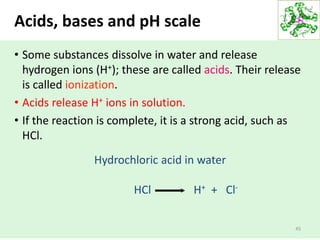
- Water dissociates into hydronium and hydroxide ions, maintaining a neutral pH through buffer systems which are important for cellular reactions.












![41
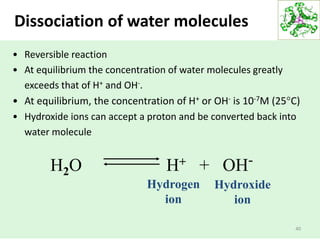
• The ionization of water can be analyzed quantitatively.
• The concentrations of the reactants and the products at equilibrium:
The ratio of these concentrations defines the equilibrium constant
(Keq).
In case of water ionization: Keq= [H+] [OH-]
[H2O]
The concentration of water at equilibrium:
the mass of 1 liter of water is 1000g
And the mass of one mole of water is 18g
the pure water has a concentration of: 1000g/l = 55.5 mole/l or
18g/mole
= 55.5 M
Dissociation of water molecules](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-41-320.jpg)
![42
Keq = [H+] [OH-]
[H2O]
Keq(55.5 M) = [H+] [OH-]
The Keq for the ionization of water has been determined under
standard conditions of pressure (1 atm) and temperature (25°C)
(1.8x10-16 M)(55.5 M) = [H+] [OH-]
1.0 x 10-14 M2 = [H+] [OH-]
Its value is: Keq= 1.8x10-16 M (the electrical conductivity
of pure water)
Dissociation of water molecules](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-42-320.jpg)
![43
Because according to the chemical equation for
dissociation H+ and OH- must have equal concentrations in
pure water, then
Kw(ion product of water)=
[H+][OH-]=[H+]2=1.0 x 10-14 M2
[H+]=1.0 x 10-7 M
[H+]= 10-7 M =[OH-]
Dissociation of water molecules
Hydrogen ion concentrations expressed in exponential form are
difficult to work with. A more useful terminology is pH, defined
as the negative logarithm of the [H+].](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-43-320.jpg)
![When [H+] = [OH-]
The solution is said
Neutral
When [H+] > [OH-] Acidic
When [H+] < [OH-] Basic
44
• The pH of a solution will depend little on the hydrogen
ions generated by the self-dissociation of water, but
rather on the presence of other substances (acids or
bases) that increase or decreases the H+ concentration.
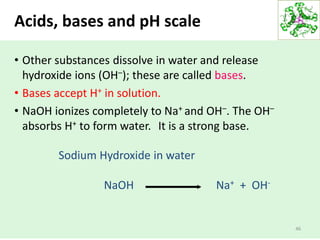
• Acids and bases are chemical substances that change
the ionic properties of solutions.](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-44-320.jpg)
![pH is a negative
logarithmic
expression of
[H+]
49](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-49-320.jpg)
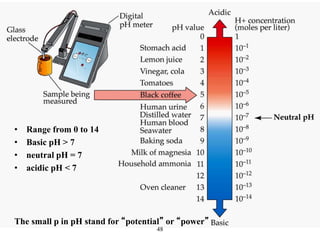
![50
• In pure H2O, [H+] and [OH-] = 10-7 M
pH = - log [H+]
So the pH of pure water is 7 Neutral
Acids, bases and pH scale](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-50-320.jpg)
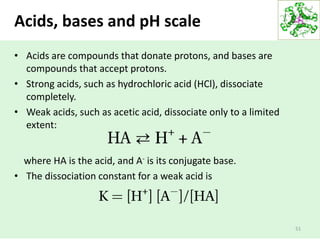
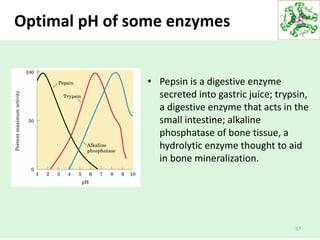
![52
Weak acids and the acid
dissociation constant (Ka)
• The stronger the acid, the lower the pKa the stronger the
base, the higher its pKa.
• The pKa can be determined experimentally; it is the pH at
the midpoint of the titration curve for the acid or base.
Ka = [H+][CH3COO-]
[CH3COOH]
pKa is a measure of acid strength](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-52-320.jpg)
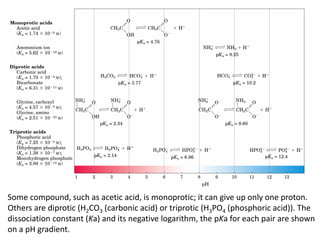
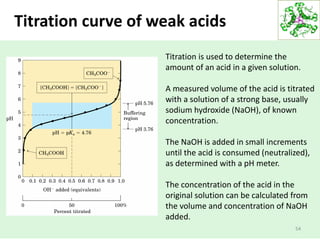
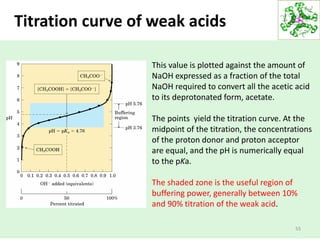
![Buffers
58
Water has a very small [H+] (10-7).
Adding just a little bit of acid or base can change the pH
drastically.
Add 0.001 M HCl: pH goes from 7 to 3!
For many applications this sensitivity is undesirable.
One of the best ways to prevent pH swings is buffering:
the use of a mixture of a weak acid and its conjugate
base (which will be a weak base).](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-58-320.jpg)
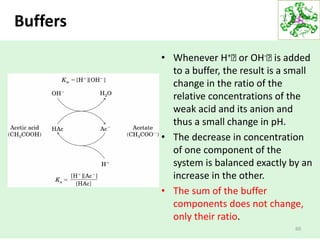
![Buffers
59
How can pH changes be minimized?
• Buffers
– Substances that minimize changes in [H+] in
solution
– Present in all biological fluids
• Human blood maintained at pH 7.4
How do buffers work?
• accept H+ ions from the solution when in excess
• donate H+ ions to the solution when depleted](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-59-320.jpg)
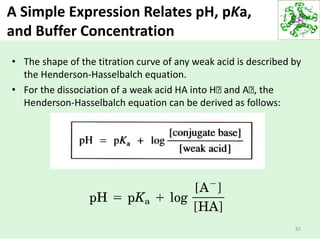

![Summary
1. Water, a nonlinear, polar molecule, serves at least three
functions in the cell: It is an effective solvent, it is a reactant
molecule, and it is a temperature buffer. As a solvent, water is
able to dissolve biomolecules that are ionic and polar.
2. The most important reaction of water is its reversible ionization
to generate proton (H+) and the hydroxide ion (OH-). The extent
of ionization is quantified by the pH scale (pH = -log [H+]).
3. The strength of an acid is defined its pKa, the negative log of its
dissociation constant.
4. Blood and other cellular fluids are maintained at a constant pH
by natural buffer systems.
64](https://image.slidesharecdn.com/lecture4-5-180915113143/85/Lecture-4-5-64-320.jpg)