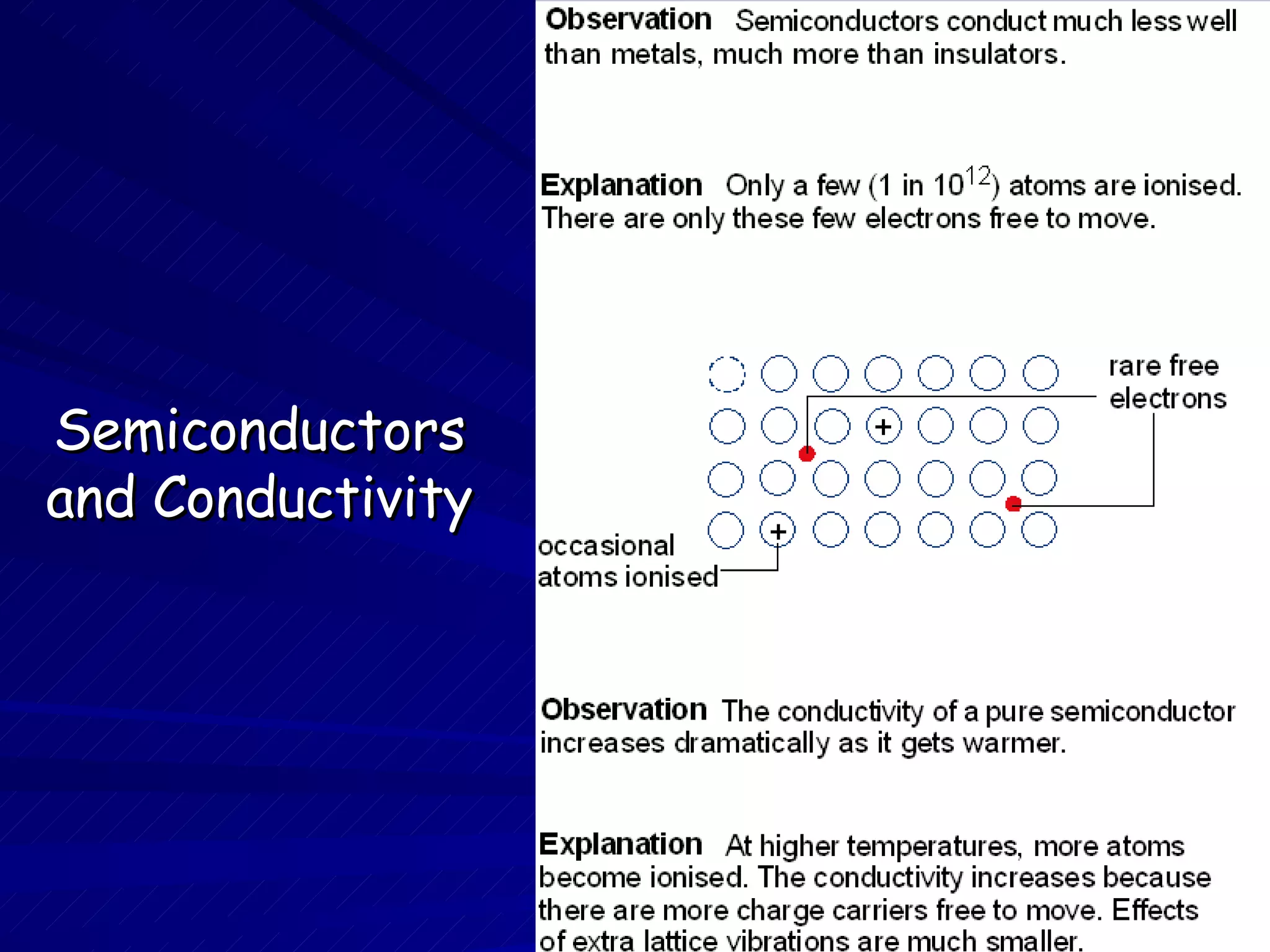
The document discusses the atomic structure and properties of metals, insulators, and semiconductors. Metals conduct electricity well due to their free electrons, are shiny as electrons scatter light, and are stiff yet ductile from electron bonding. Insulators like ceramics and polymers do not conduct as their electrons are locked in ionic or covalent bonds, making them stiff or brittle. Semiconductors have properties between metals and insulators with fewer free electrons. Their conductivity increases with temperature unlike metals but can also be controlled through doping with other atoms to donate or accept free electrons.