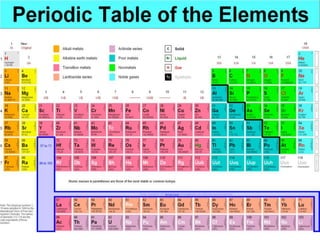
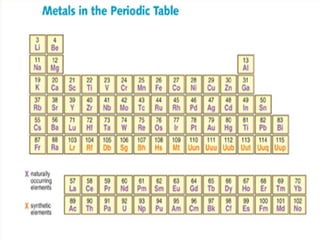
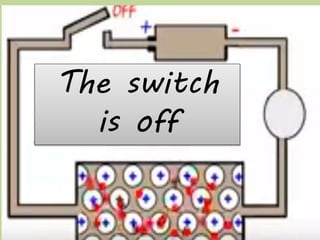
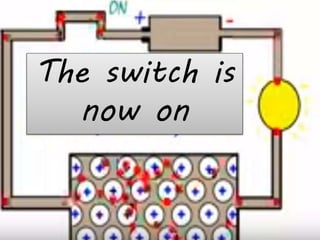
Metals are typically hard, shiny materials that conduct heat and electricity well. They exist as solids at room temperature, except for a few like mercury. Metals reflect light well due to their loosely bound outer electrons. Other key properties include malleability, ductility, conductivity, density, and high melting and boiling points. Metals conduct electricity and heat well because their molecular bonding allows free electrons to move freely within their structure when a current is applied.