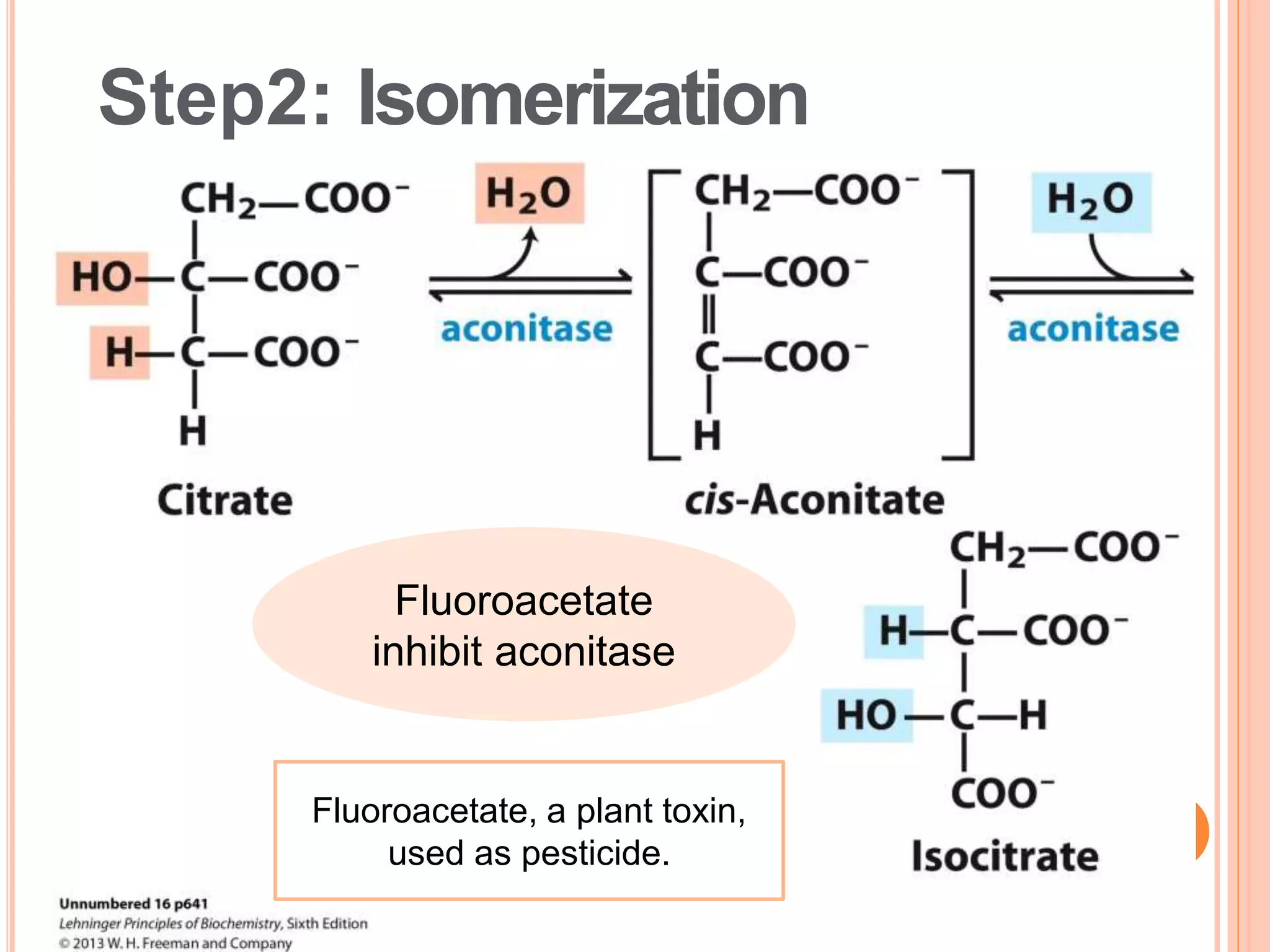
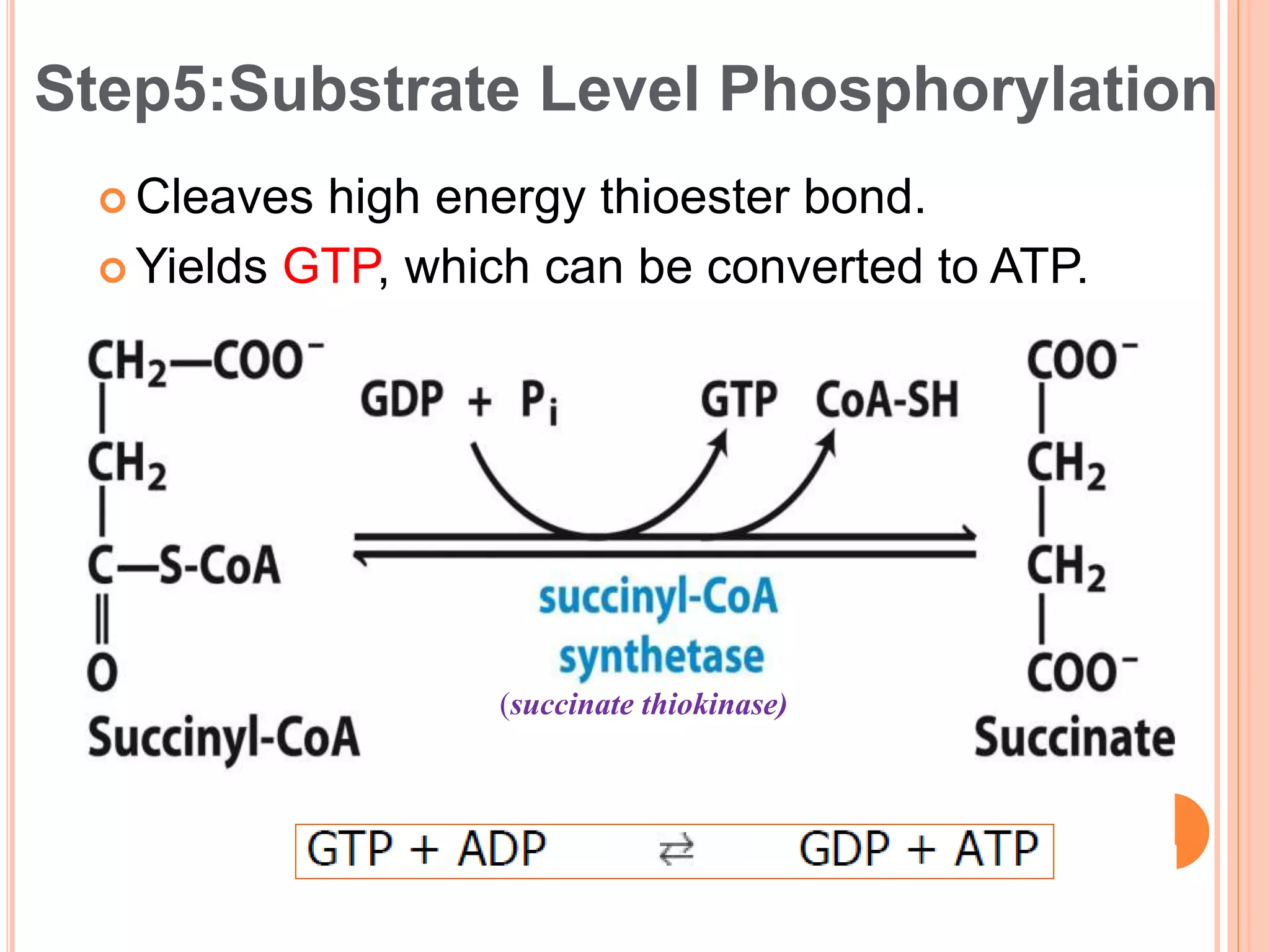
The document discusses glycolysis and the citric acid cycle. Glycolysis involves 10 steps that break down glucose and generate a small amount of ATP without oxygen. The citric acid cycle is a series of chemical reactions in the mitochondria that further oxidizes pyruvate from glycolysis to extract more chemical energy. It involves 8 steps that produce carbon dioxide, NADH, and FADH2 to fuel the electron transport chain for oxidative phosphorylation to generate large amounts of ATP. Both pathways are tightly regulated and provide precursors for other biological processes.






























![ The Citric acid cycle (Tricarboxylic acid cycle [TCA
cycle] or Krebs cycle) plays several roles in
metabolism.
Final common pathway for oxidation of fuel
molecules such as carbohydrates, amino acids,
and fatty acids.
Provides energy: ATP.
Aerobic pathway, O2 is required.
Occurs totally in the mitochondria.
Citric Acid Cycle](https://image.slidesharecdn.com/4-230517232708-e4499dab/75/4-2-glycolysis-TCA-cycle-ppt-31-2048.jpg)