Lipid Metabolism
The document discusses lipid metabolism, including:
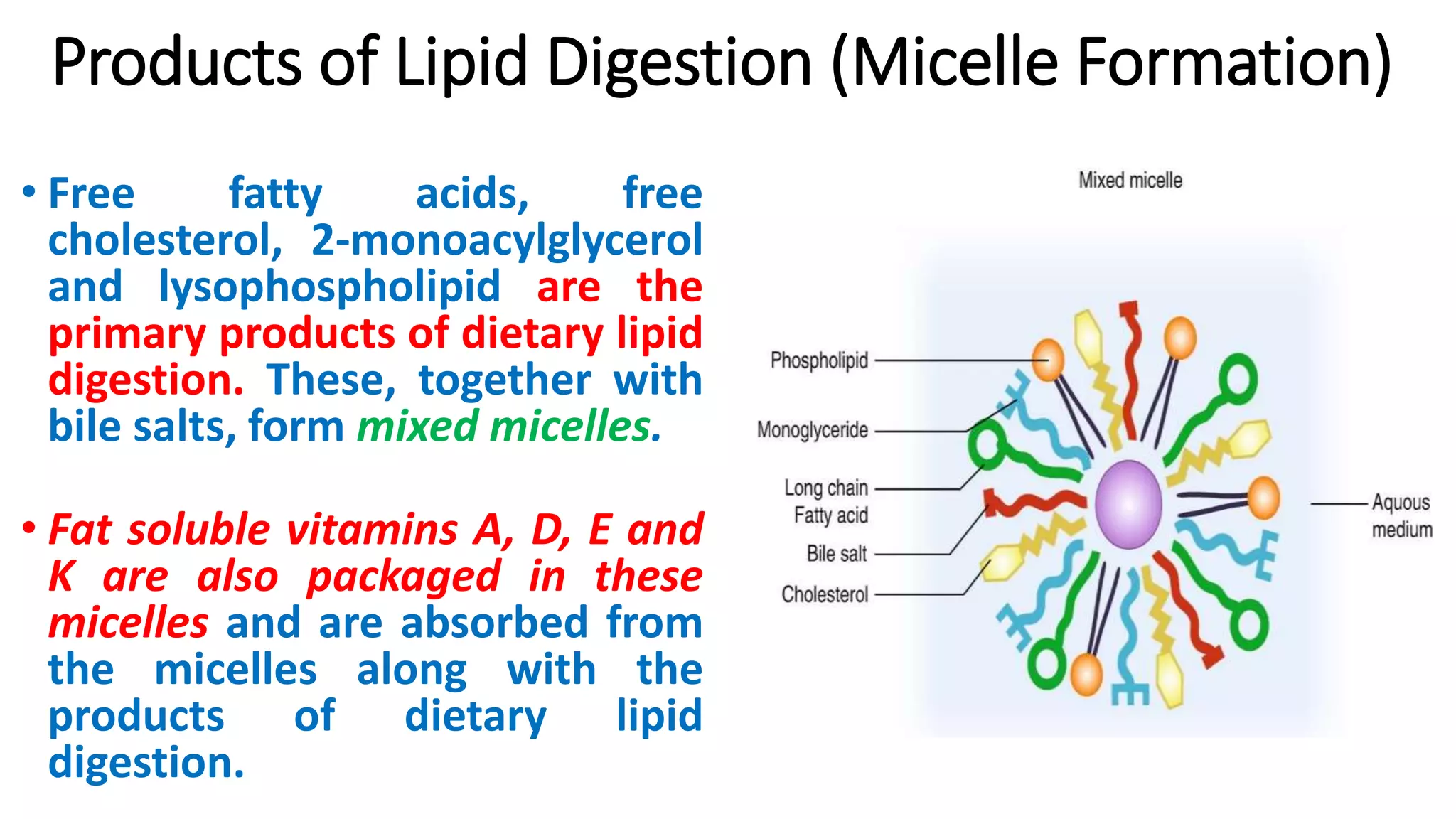
1. Lipids are digested in the small intestine by pancreatic enzymes and emulsified into micelles.
2. The products of digestion including fatty acids are absorbed into the blood as chylomicrons or transported to the liver.
3. In the liver and other tissues, fatty acids undergo beta-oxidation to generate acetyl-CoA for energy production or ketone bodies during fasting.