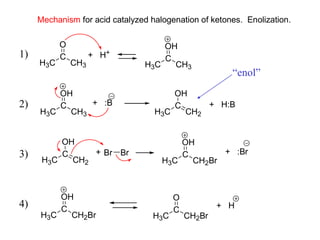
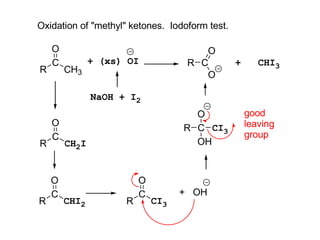
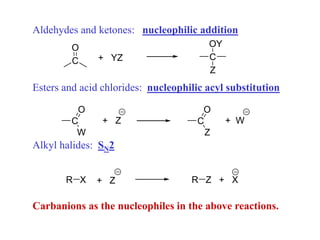
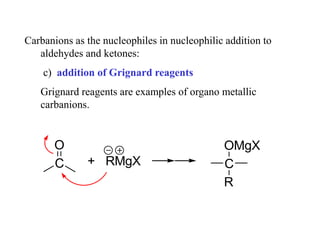
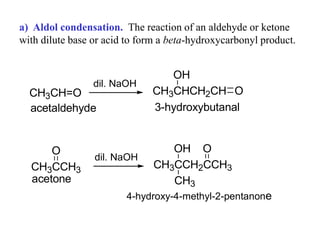
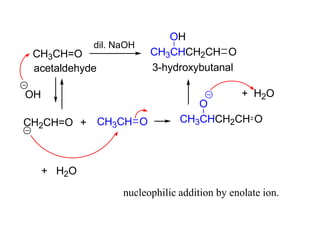
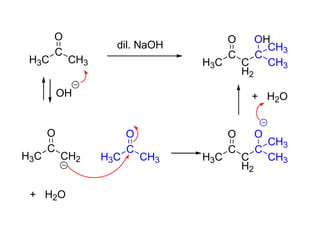
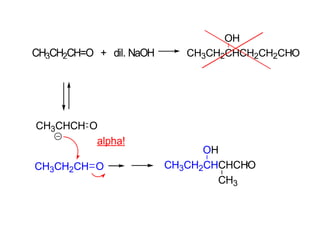
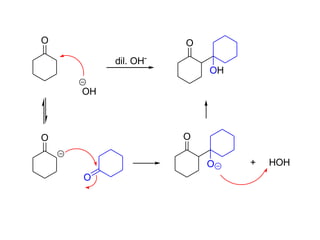
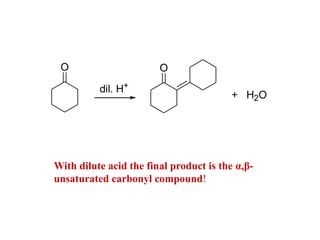
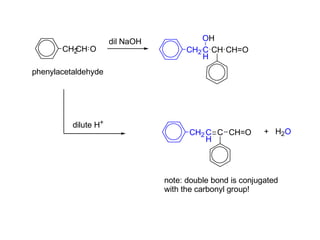
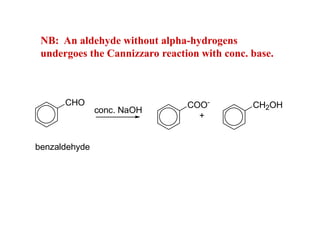
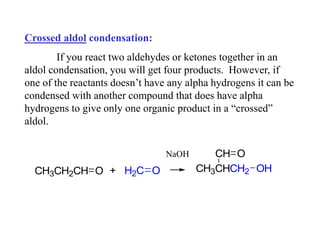
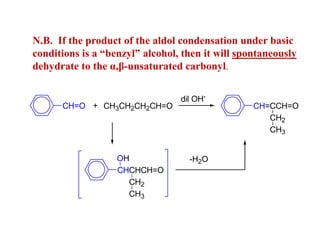
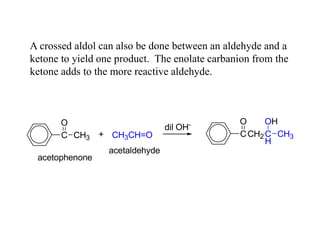
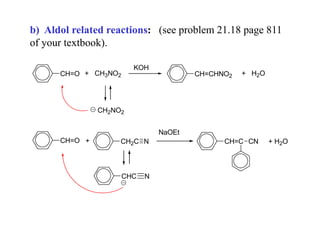
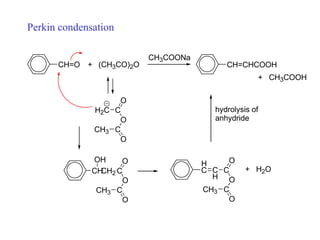
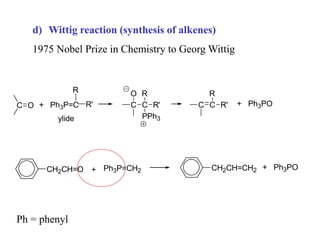
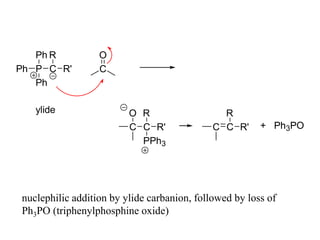
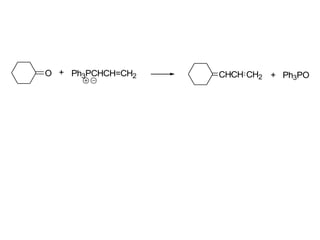
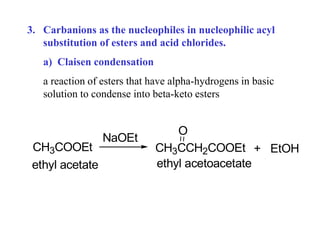
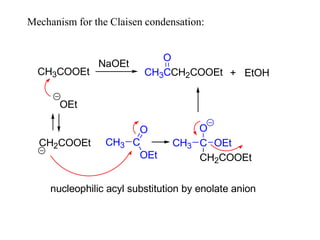
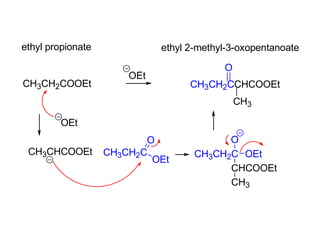
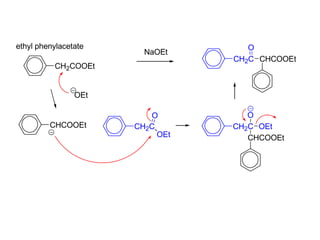
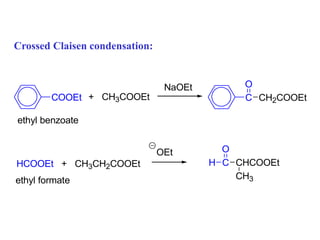
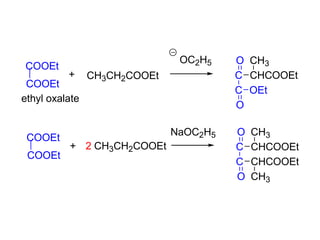
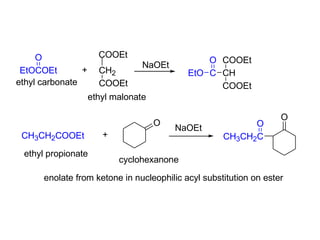
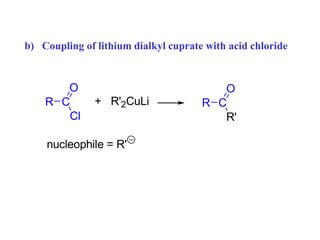
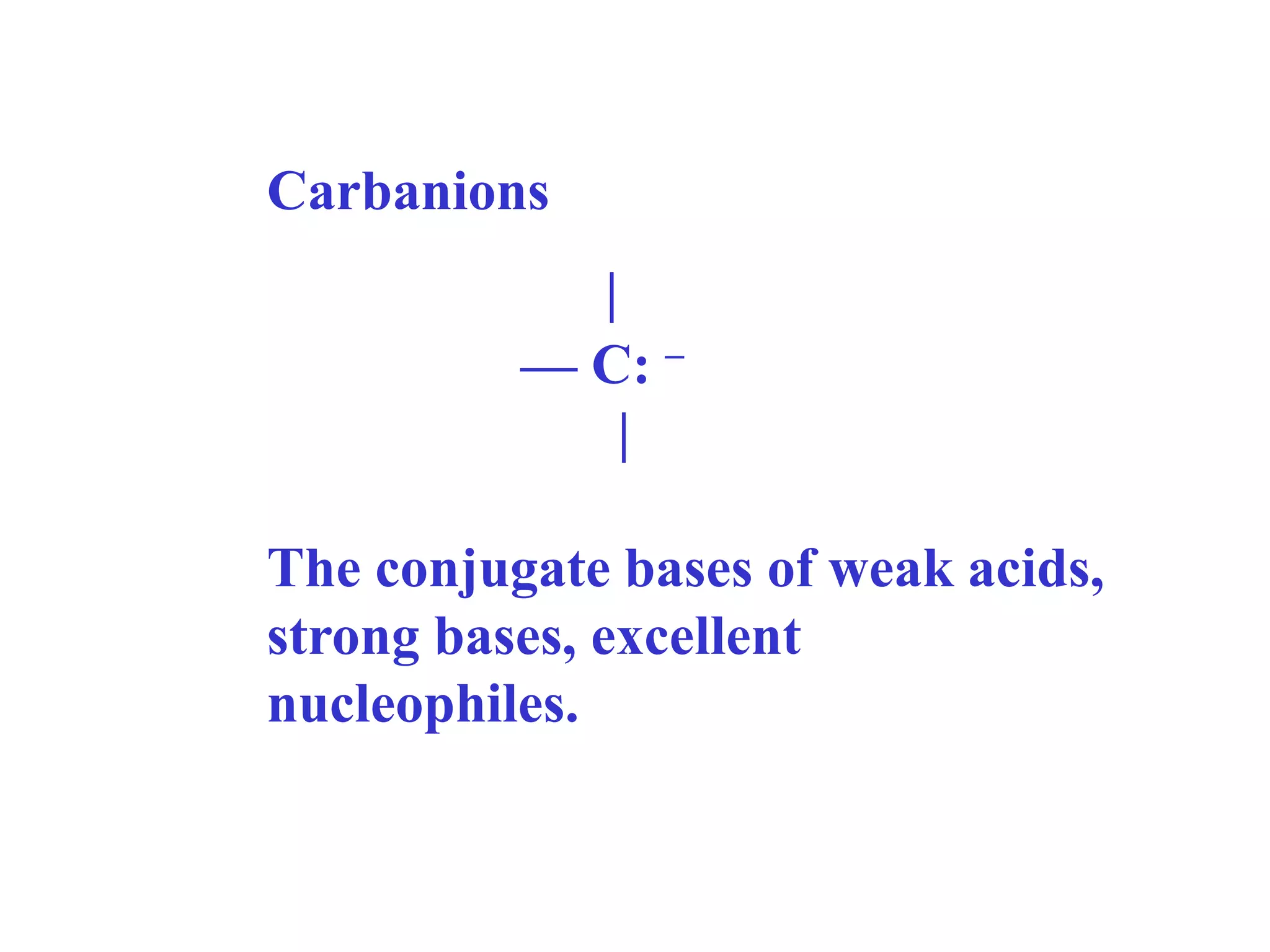
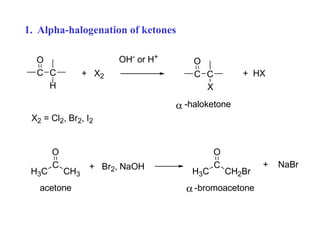
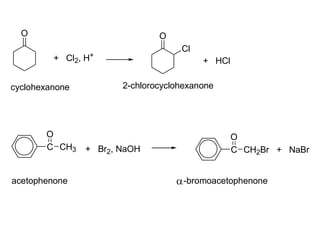
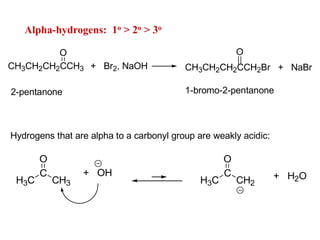
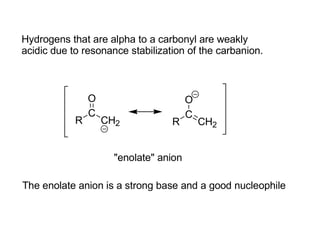
Carbanions are the conjugate bases of weak acids and are strong bases and good nucleophiles. They react in several substitution and addition reactions including: 1) alpha-halogenation of ketones, 2) nucleophilic addition to aldehydes and ketones such as aldol reactions, 3) nucleophilic acyl substitution of esters and acid chlorides such as Claisen condensations, 4) SN2 reactions with alkyl halides, and 5) Michael additions to α,β-unsaturated carbonyls.
![Mechanism for base promoted alpha-bromination of acetone:
H3C
C
CH3
O
H3C
C
CH2
O
+ OH + H2O
RDS
H3C
C
CH2
O
+ Br Br
H3C
C
CH2Br
O
+ Br
1)
2)
Rate = k [acetone] [base]](https://image.slidesharecdn.com/carbanions-230620090326-580eca20/85/CARBANIONS-ppt-6-320.jpg)