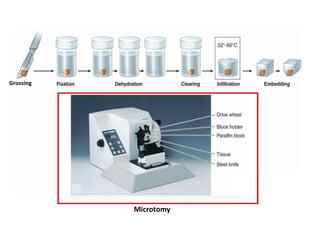
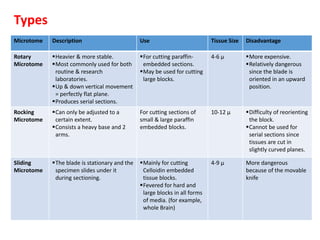
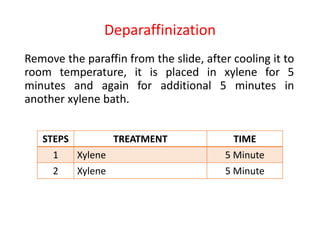
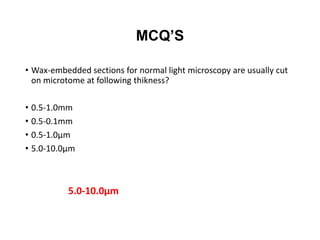
The document provides a comprehensive overview of microtomy, including its purpose, different types of microtomes, their components, care and maintenance, as well as procedures for sectioning and staining tissues. It discusses various microtome types such as rotary, rocking, sliding, and ultrathin microtomes, highlighting their specific uses and limitations. Additionally, it outlines the processes involved in preparing tissue samples for microscopy, from embedding to staining, and concludes with references for further reading.