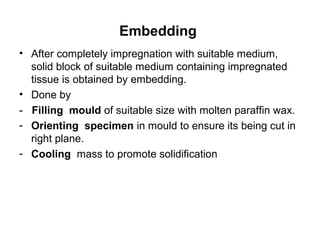
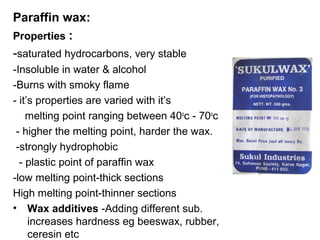
The document discusses various techniques used in histopathology sample processing including decalcification, fixation, dehydration, clearing, embedding and sectioning. It covers different chemical agents used for each step along with their properties and advantages. Various methods are described such as paraffin, celloidin and vacuum embedding for optimal tissue preservation and section quality. Automatic tissue processors and freeze drying are also mentioned as techniques to reduce processing time.


![Methods of decalcification:
• Chemical method
-Acid reagents
-Nitric acid- 4hrs
-Trichloracetic acid- 48hrs for small
-Formic acid- 12-24hrs
Chelating agents:
Ethylene Diamine Tetra acetic acid[EDTA]- 4-40 days
• Ion–exchange resin method
( ammonium form of sulphonated polysterene resin)
• Electrophoretic method](https://image.slidesharecdn.com/16histotechniques2-121125061150-phpapp01/85/16-histotechniques-2-3-320.jpg)