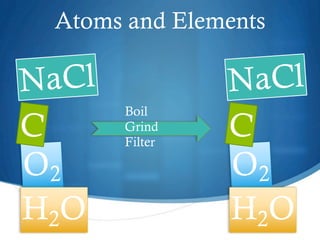
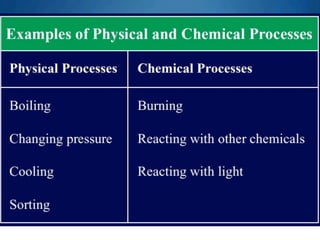
This chapter discusses different types of substances and mixtures. It defines a substance as matter that has a fixed composition and cannot be separated into simpler substances by physical processes like boiling or filtering. Compounds have a fixed ratio of atoms, while mixtures can have varying proportions and their components can be separated. Solutions are homogeneous mixtures that are evenly mixed on a molecular level. The chapter also describes how solutions form and the different types of solutions.