1. A mixture is a combination of two or more substances that are not chemically combined and where each substance retains its own chemical properties.
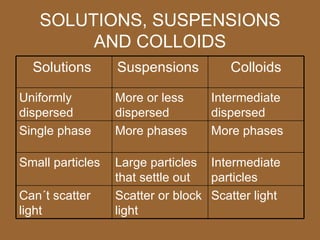
2. There are three main types of mixtures: homogeneous mixtures, heterogeneous mixtures, and colloids. Homogeneous mixtures are uniform throughout while heterogeneous mixtures have visually distinct components.
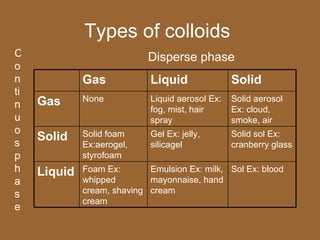
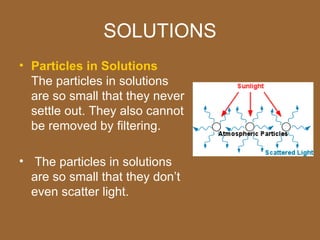
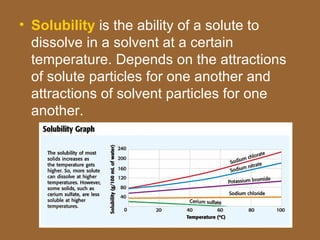
3. Important homogeneous mixtures include solutions, where a solute is evenly dissolved in a solvent, and alloys, which are solid solutions of metals. Heterogeneous mixtures include suspensions, where a liquid contains large undissolved particles.