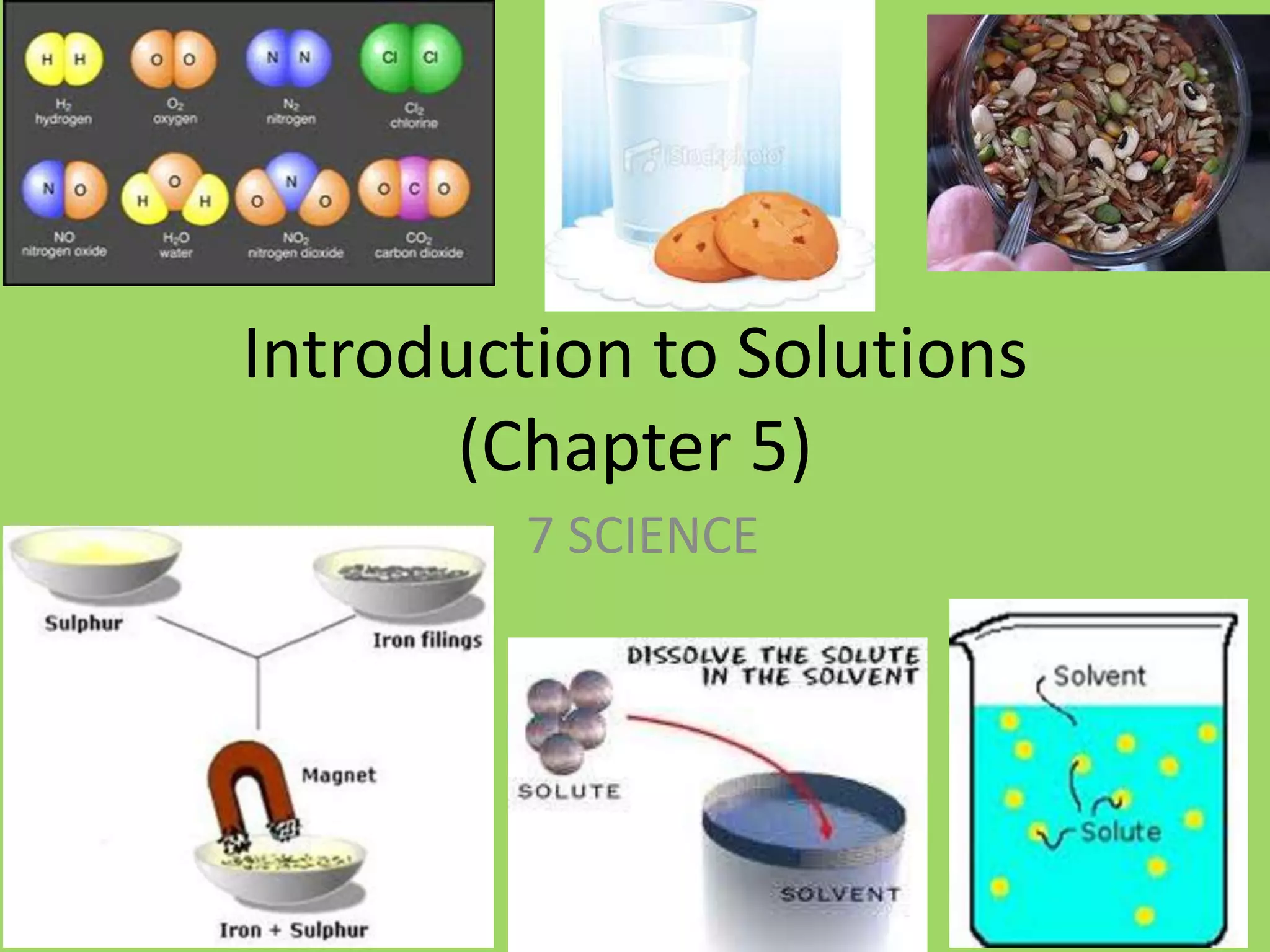
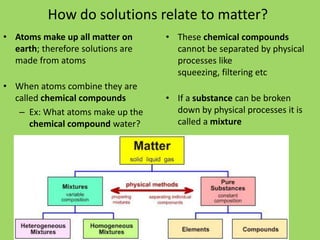
This document introduces key concepts about solutions and mixtures. It defines a solution as a homogeneous mixture formed when a solute dissolves evenly throughout a solvent. Water is discussed as a common solvent that can dissolve many substances to form solutions. The reading also distinguishes between heterogeneous mixtures, where substances are not evenly mixed, and homogeneous mixtures or solutions, where the mixing appears uniform. An upcoming student activity is described where groups will create mixtures using water and different substances to observe solubility.