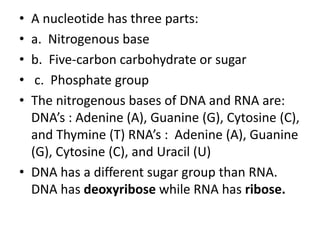
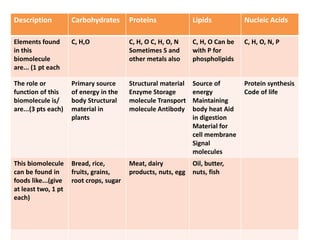
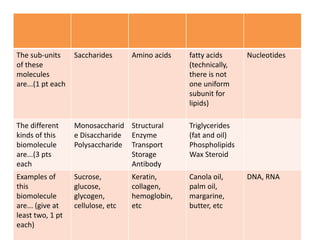
The document outlines the content standards for understanding biological macromolecules, emphasizing their structure, function, and classification. It details carbohydrates, proteins, lipids, and nucleic acids, explaining their roles in energy storage, structural formation, and genetic information transfer. Additionally, it describes specific types and examples of each macromolecule, highlighting their importance in biological processes.