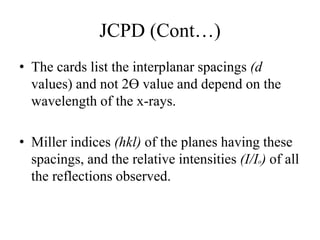
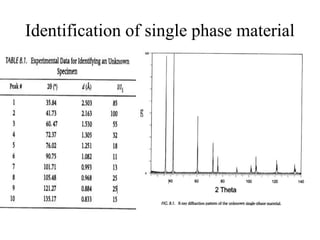
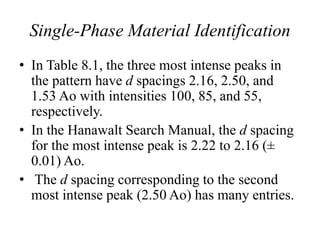
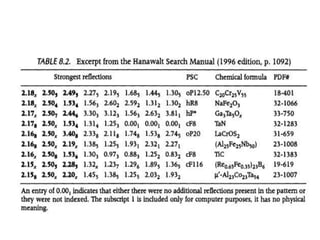
1. The document describes how to identify an unknown specimen using powder X-ray diffraction by comparing the specimen's diffraction pattern to standard patterns in the JCPDS database.
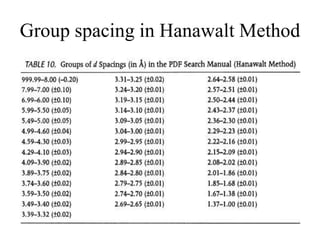
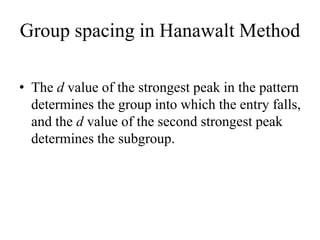
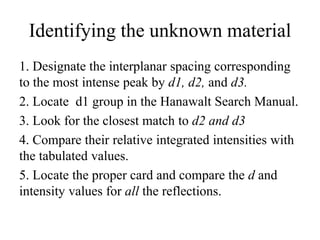
2. The identification process involves finding the three strongest peaks in the unknown pattern, locating them in the Hanawalt Search Manual index, and comparing relative intensities and all d-spacings to potential matching patterns.
3. For a single-phase specimen, once a match is found the identification is complete. For multi-phase specimens, additional peaks must be considered to distinguish between potential matching patterns.