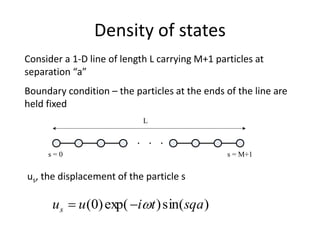
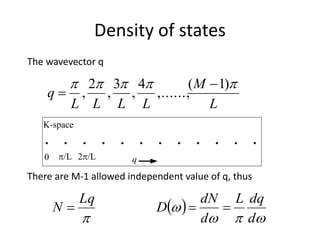
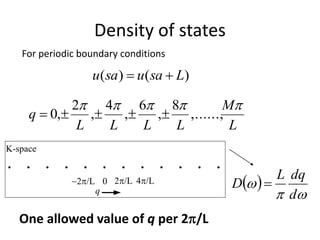
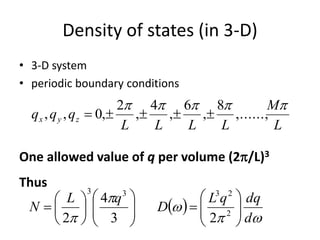
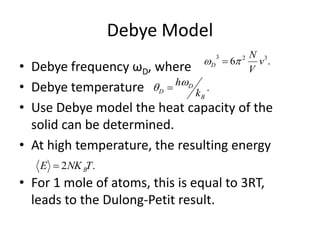
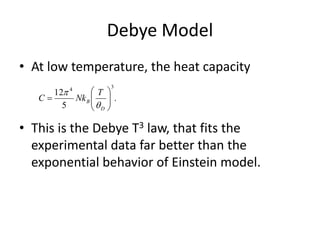
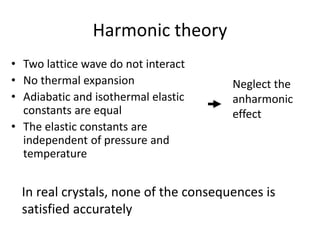
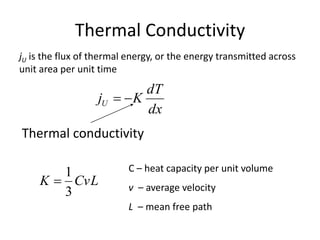
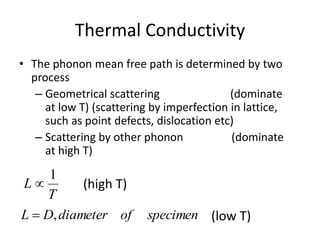
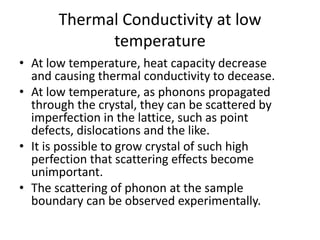
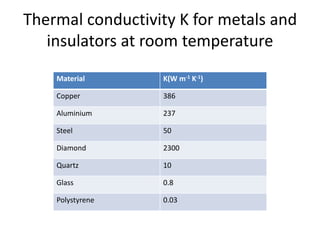
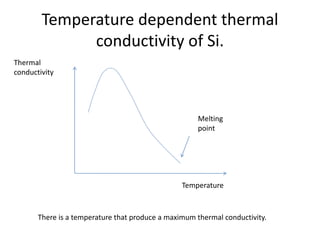
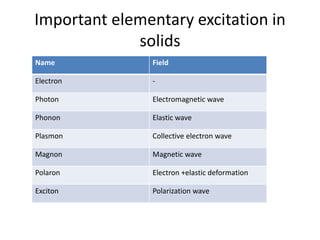
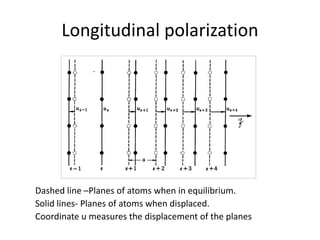
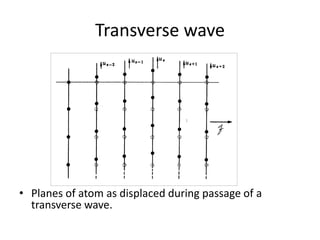
This document discusses lattice vibrations in solids. It begins by introducing different types of elementary excitations in solids including phonons, which are quantized elastic waves in a crystal. It then describes lattice vibrations as waves that propagate through the crystal as planes of atoms moving in and out of phase. Both longitudinal and transverse polarization of these waves are discussed. Equations of motion are provided and solved to show the wave-like behavior of lattice vibrations. The document also covers phonon dispersion relations, group velocity, Brillouin zones, phonons in diatomic crystals, quantization of elastic waves, phonon momentum, heat capacity of lattices, and early models like Einstein and Debye models to explain temperature-dependent


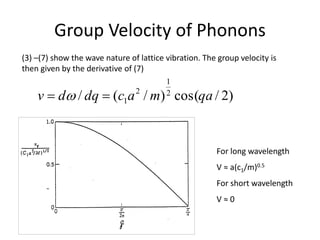
( tqxiAxu (3)
ω is the angular frequency
q is the wavevector
P = h/2 x q
E = h/2 x ω
The vibrational motion in the crystal is regarded as a kind of wave
propagation. Because of the finite extent of the crystal, the wave motion is
often regarded as a standing wave system. the phonon is defined to be
the quantised energy of elastic waves in a crystal. The phonons are similar
to the photons in electromagnetic waves in many respects. Both of these
two excitations exhibit the duality property, namely possessing both wave-
like and particle-like behaviour.](https://image.slidesharecdn.com/crystaldynamics-150916170117-lva1-app6891/85/Crystal-dynamics-7-320.jpg)
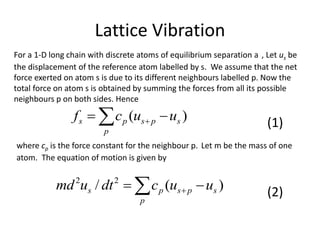
![Lattice Vibration
Remember that we are dealing with discrete atoms of equilibrium
separation a. Thus for any general atoms with label s + p, x should be
replaced by (s + p)a.
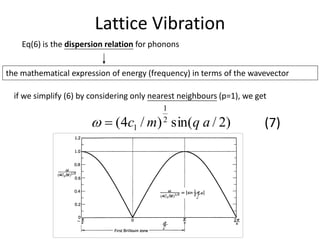
)exp(])(exp[ tiqapsiuu ps (4)
Substituting this solution (4) in the equation of motion, we obtain
]1)[exp(2
ipqacm pp (5)
for the lattice with inversion symmetry about the origin, (5) may be
re-written as
)]cos(1[)/2(2
pqacm pp (6)](https://image.slidesharecdn.com/crystaldynamics-150916170117-lva1-app6891/85/Crystal-dynamics-8-320.jpg)
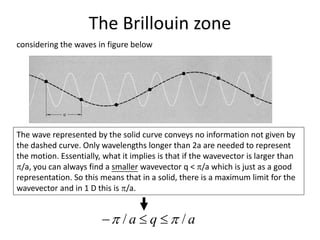
![The Brillouin zone
“if the wavevector is larger than /a, you can always find a smaller
wavevector q < /a which is just as a good representation”
How ??
Consider two wavevector q and q + n(2π/a) where n is an integer
)exp()2exp()exp(
])/2(exp[
iqaniiqa
aanqi
)/2(' anqq
q outside the first zone
q' the corresponding value inside the zone](https://image.slidesharecdn.com/crystaldynamics-150916170117-lva1-app6891/85/Crystal-dynamics-12-320.jpg)
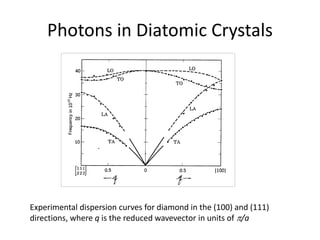



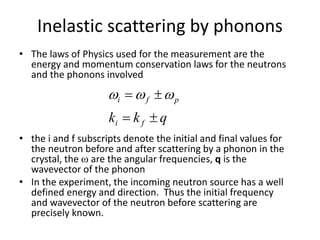
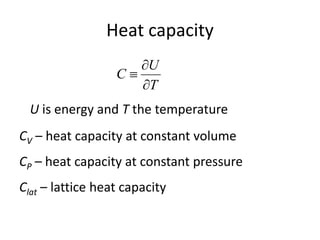
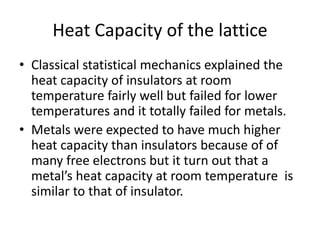

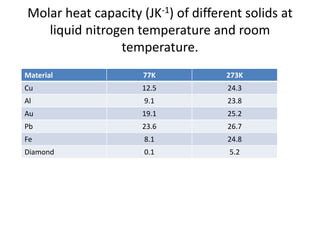
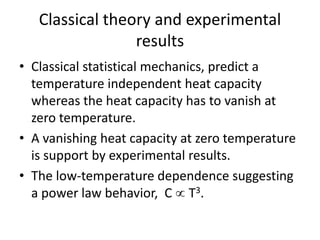
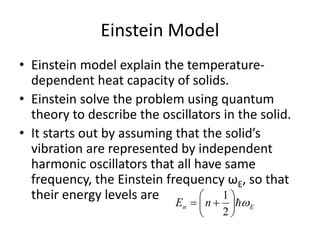
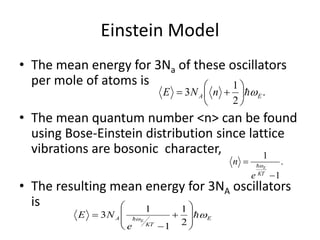
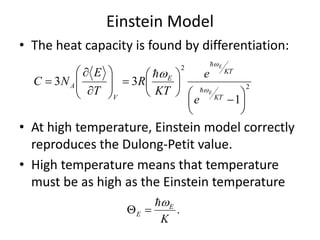
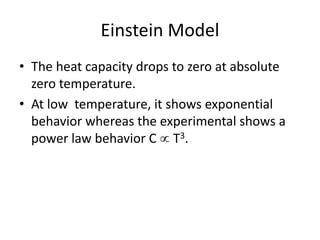
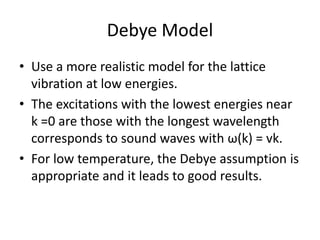
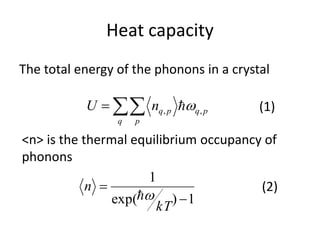
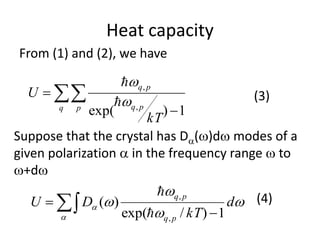
![Heat capacity
d
dN
D )(
Density of states
(The number of modes per
unit frequency range)
lattice heat capacity
a
a d
kT
kT
kT
Dk
T
U
C
pq
pq
pq
lat
2
,
,
2
,
]1)/[exp(
)/exp(
)(
](https://image.slidesharecdn.com/crystaldynamics-150916170117-lva1-app6891/85/Crystal-dynamics-33-320.jpg)