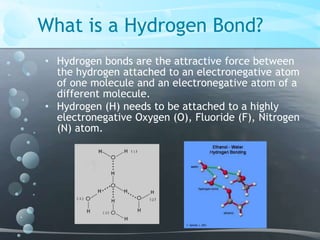
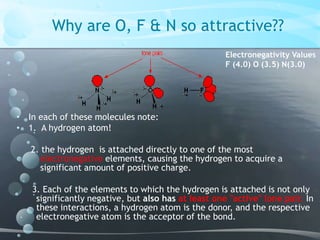
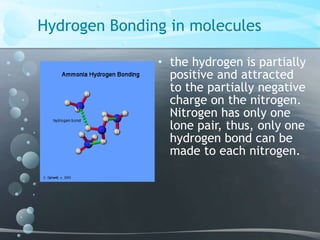
1. Hydrogen bonding occurs between hydrogen atoms attached to electronegative atoms like oxygen, fluorine, and nitrogen of one molecule and an electronegative atom of another molecule.
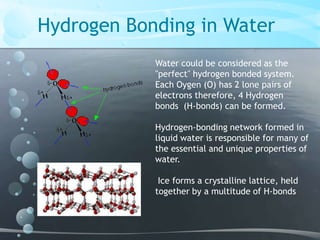
2. Water is able to form extensive hydrogen bonding networks between molecules due to each water molecule having two hydrogen atoms and two lone pairs of electrons on the oxygen atom.
3. The hydrogen bonding network in liquid water is responsible for its unique properties, while hydrogen bonding in ice forms its crystalline lattice structure.