Embed presentation
Download to read offline







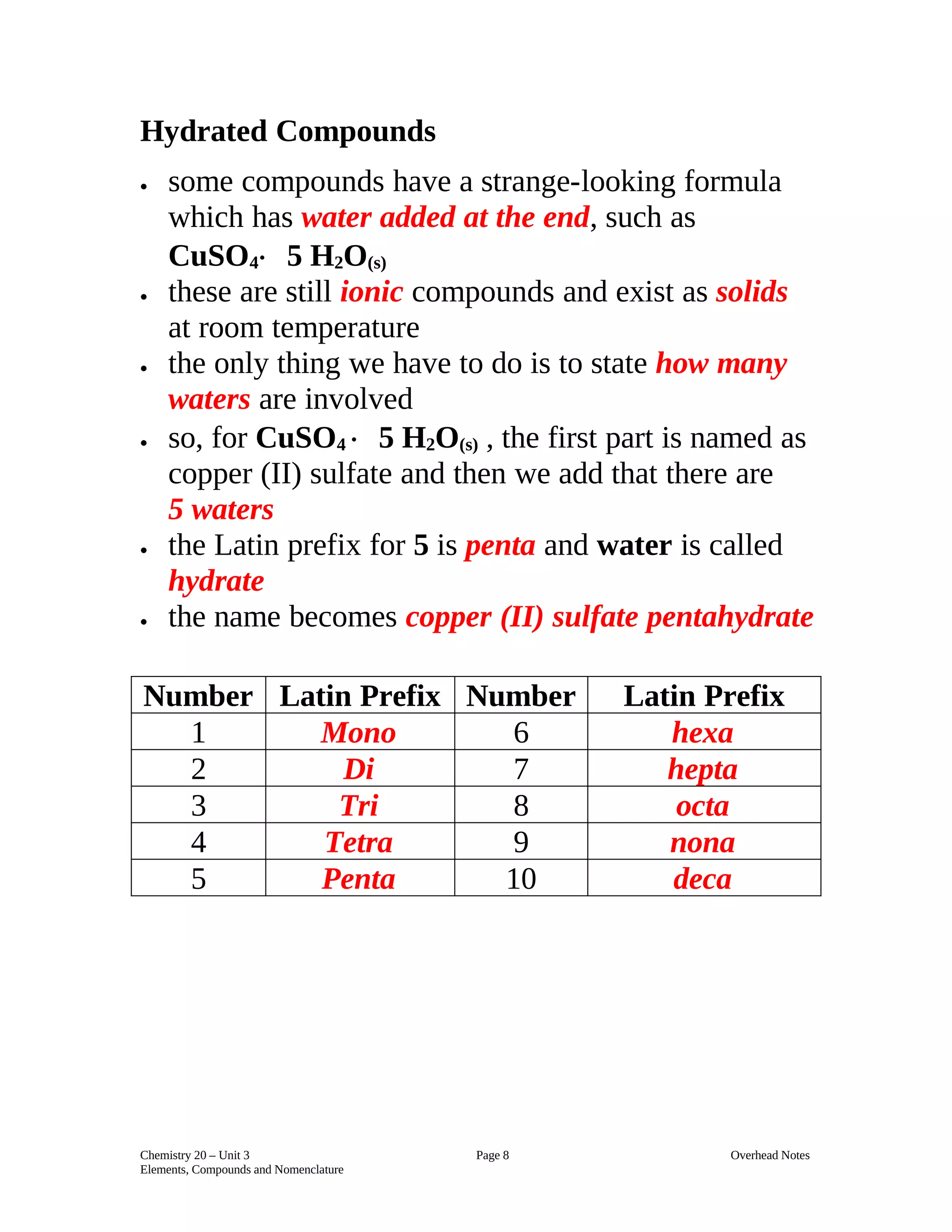





1) The document discusses chemical nomenclature and naming conventions for various types of substances including pure elements, ionic compounds, molecular compounds, and acids. 2) For ionic compounds, the first element is given its normal name and the second element takes an "ide" ending, and roman numerals are used to indicate charge for polyatomic ions. Hydrated compounds also use Latin prefixes to describe the number of water molecules. 3) Molecular compounds use prefixes to describe the number of each element and classical names are also common. Hydrogen compounds that dissolve in water form acids, following specific naming conventions based on the anion present.












