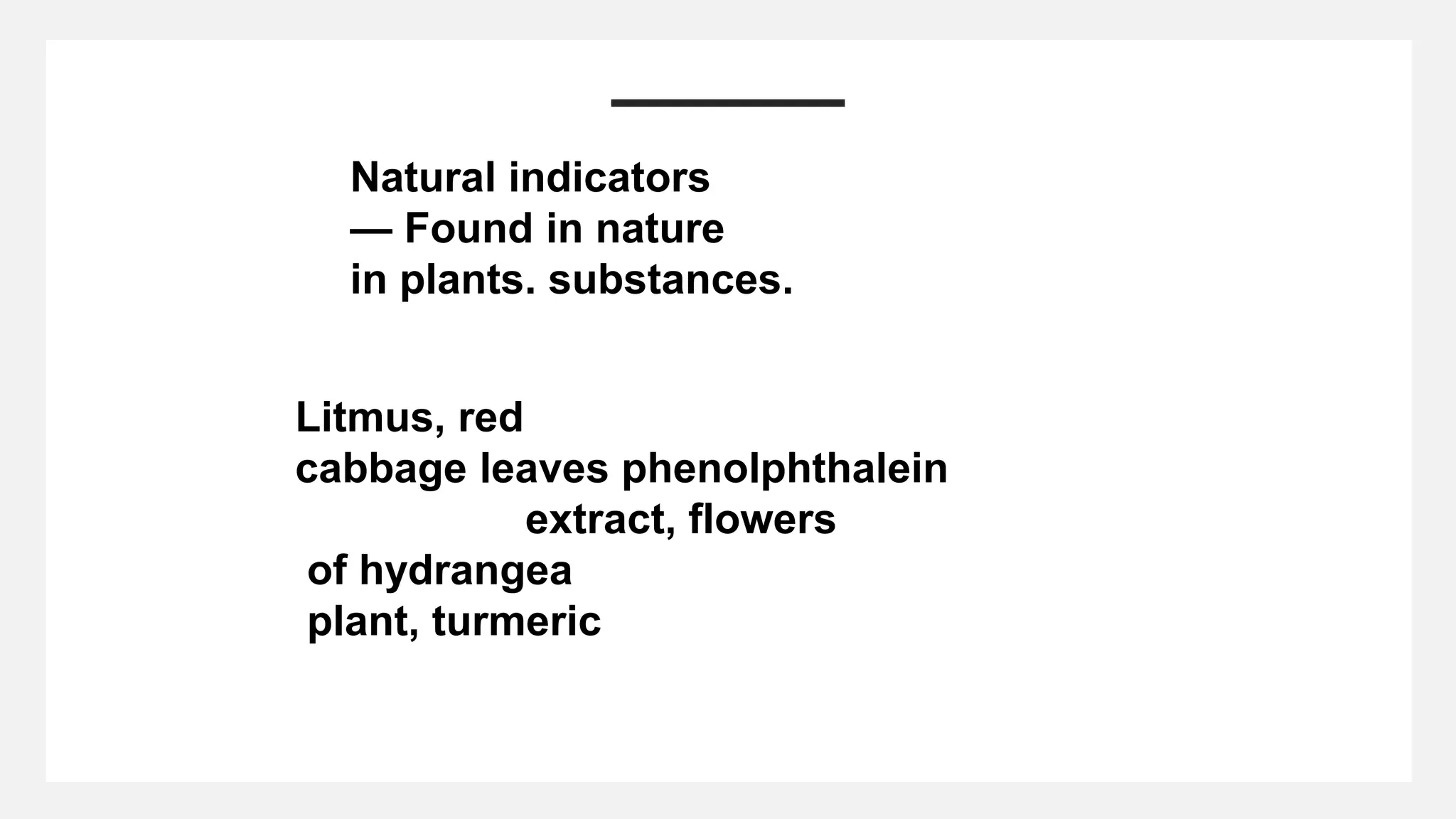
The document covers the properties and reactions of acids, bases, and salts, detailing how acids produce hydrogen ions in water, taste sour, and turn blue litmus red. It lists various reactions between acids and metals, carbonates, and bicarbonates, along with the characteristics of bases that are bitter and turn red litmus blue. The document also explains the role of indicators in identifying acids and bases, mentioning natural and synthetic indicators.








![Reactions with Acids
1. Reaction of Acid with Metal
Acid + Metal → Salt + Hydrogen gas
Mg + H2 SO4 → H2 + Mg SO4
2. Reaction of Acid with Carbonates
Na2 CO3 (s) + 2 HCl (aq) → 2NaCl (aq) + H2O(l) + CO2(g)
3. Reaction of Acid with Bicarbonates
NaHCO3 (s) + HCl (aq) → NaCl(aq) + H2O (l) + CO2 (g)
Strong Bases : NaOH, KOH, Ca(OH) 2
Weak Bases : NH4OH
Alkalis : These are bases which are soluble
in water [NaOH, KOH, Ca(OH) 2].](https://image.slidesharecdn.com/greencactuspptforteaching-wpsoffice-200715125327/75/Acid-base-and-salt-class-10-science-what-are-indicators-10-2048.jpg)