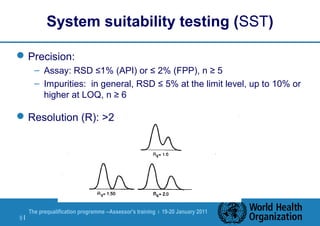
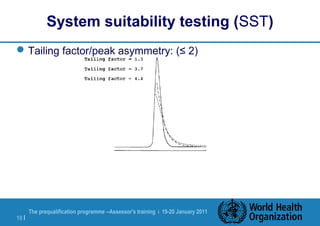
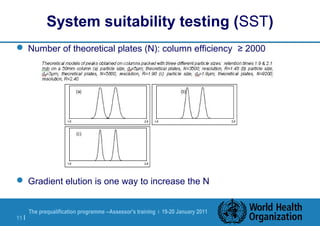
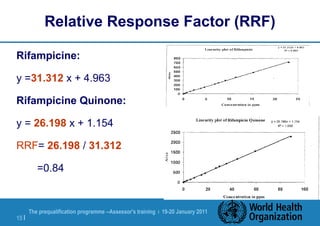
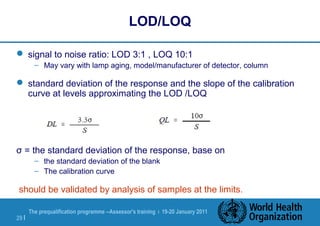
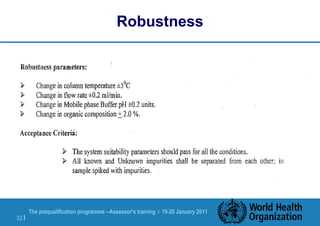
The document discusses HPLC methodology and validation basics. It outlines the key components of an HPLC test procedure including system suitability testing and relative response factors. Validation of HPLC methods is also covered, including specificity, linearity, accuracy, precision and LOD/LOQ. A case study example is provided to demonstrate how these concepts are applied. Validation is important to demonstrate that HPLC methods are suitable for their intended use and generate reliable data for product acceptance, release and stability testing.