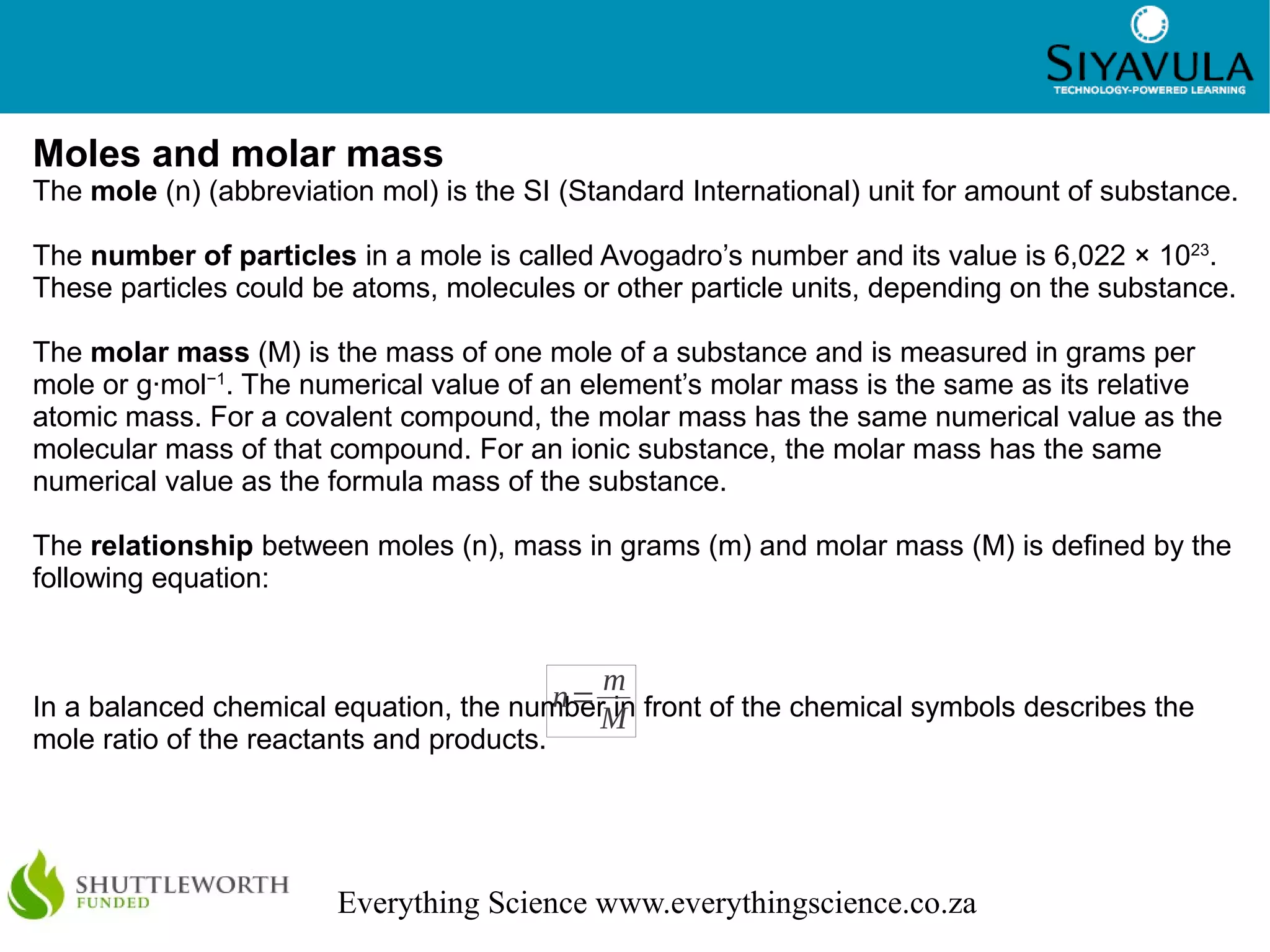
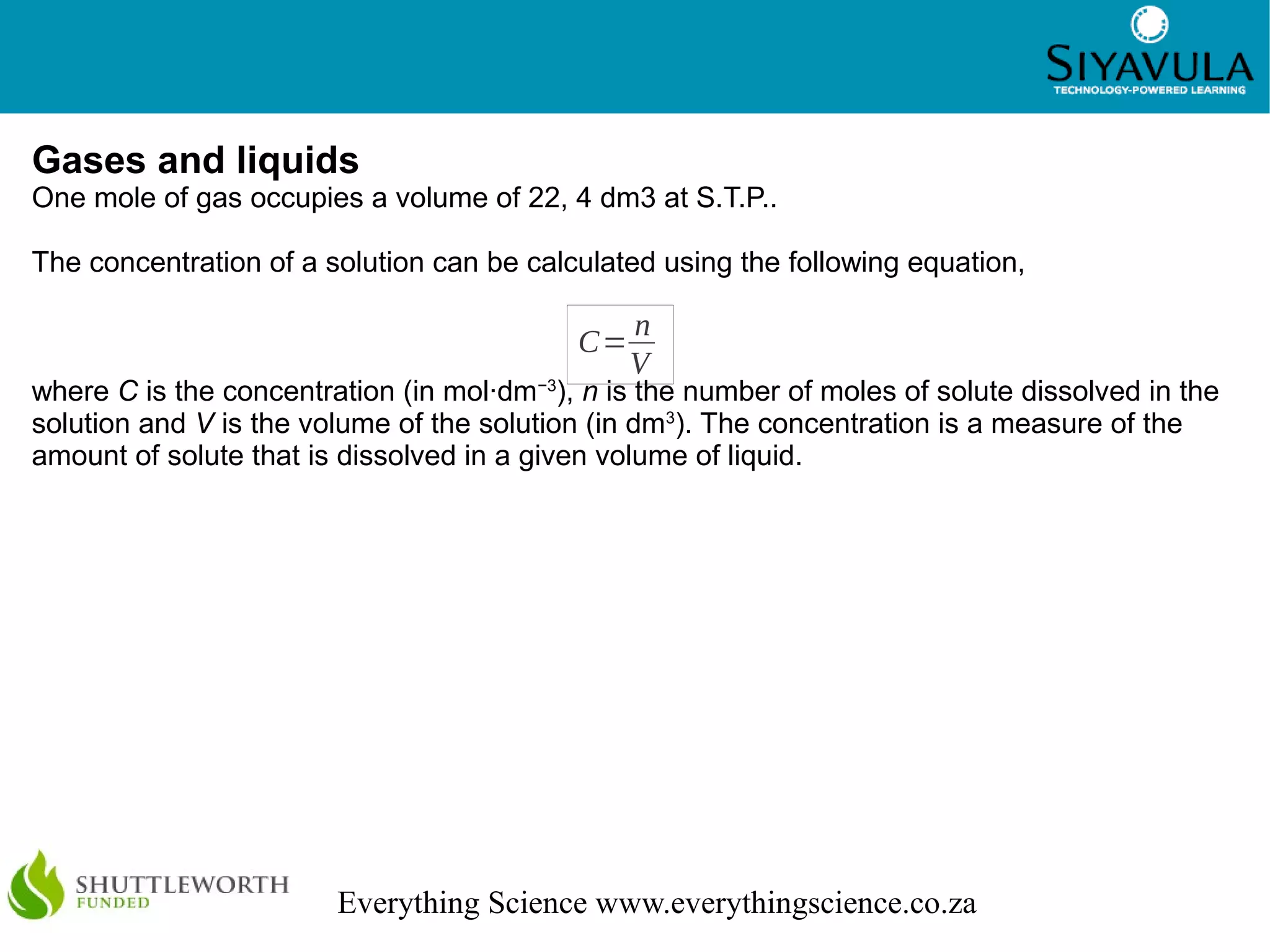
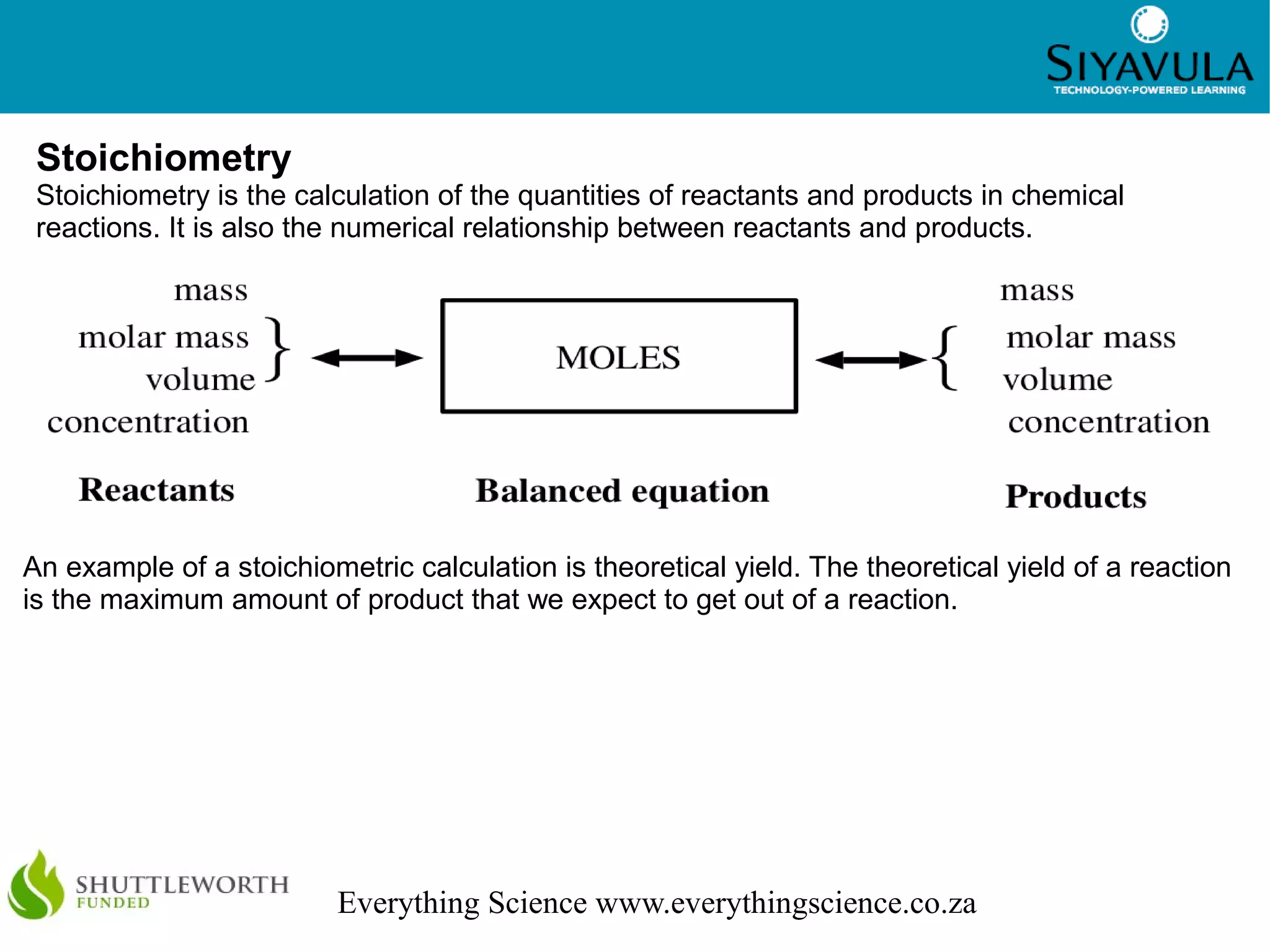
This document discusses quantitative aspects of chemical changes including moles, molar mass, calculations using moles, gases and liquids, and stoichiometry. The mole is the standard unit for amount of substance, with one mole containing 6.022 x 1023 particles. Molar mass is the mass of one mole of a substance measured in grams per mole. Stoichiometry is the calculation of quantities in chemical reactions using mole ratios and can be used to determine theoretical yields.