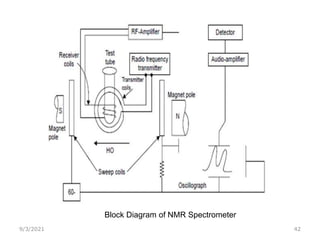
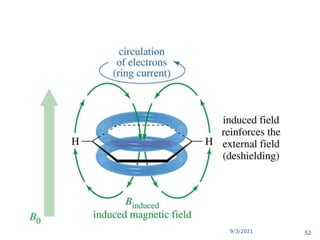
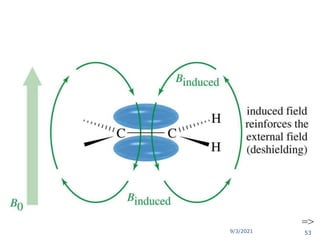
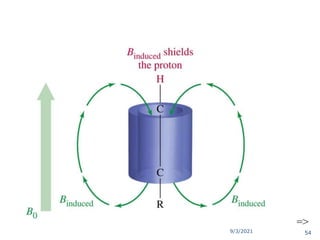
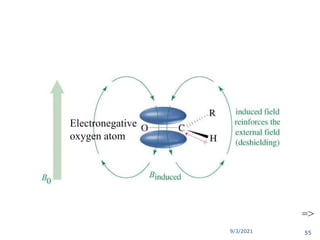
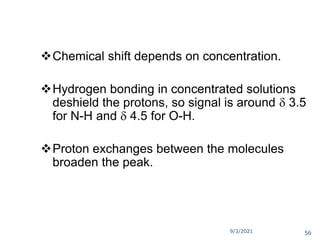
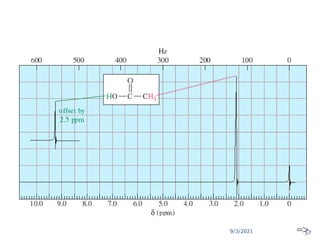
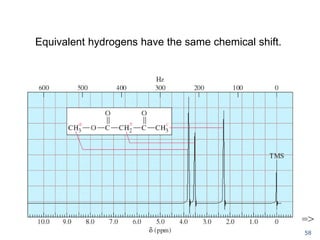
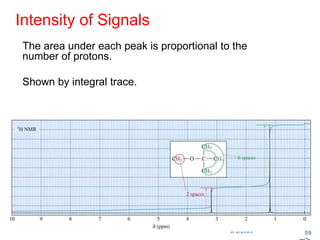
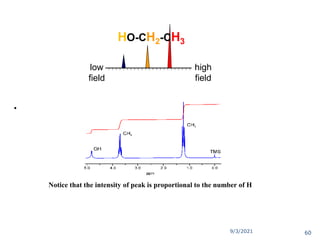
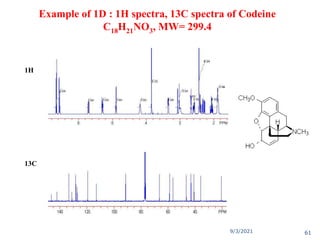
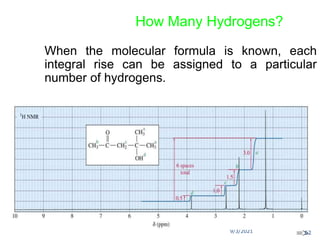
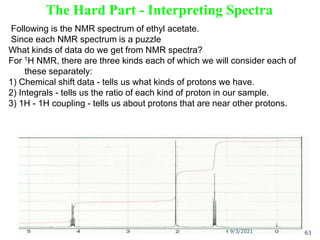
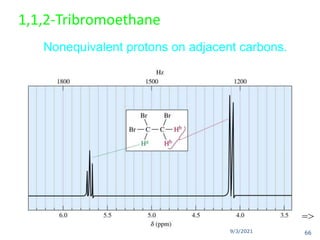
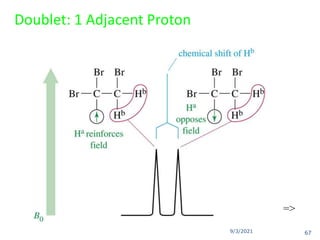
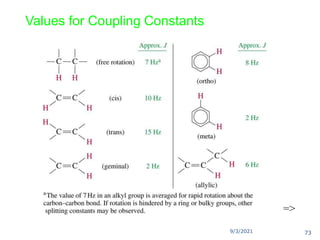
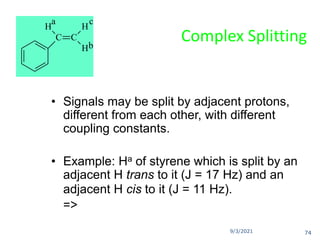
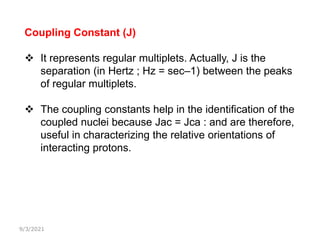
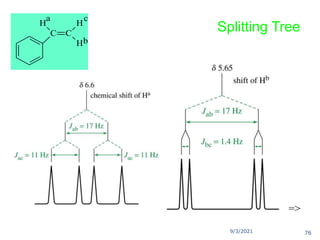
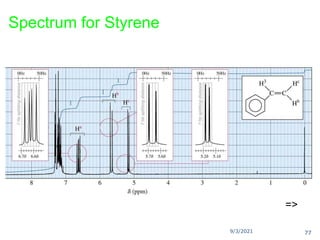
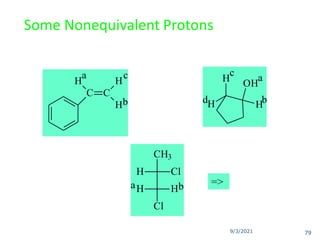
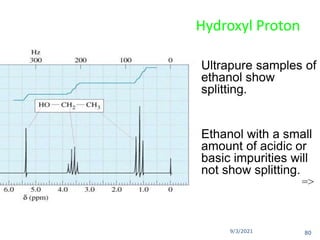
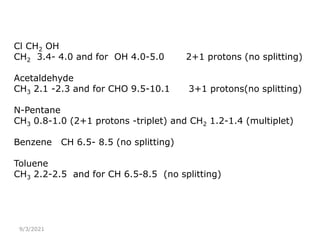
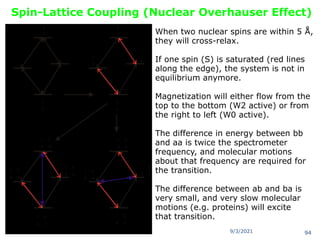
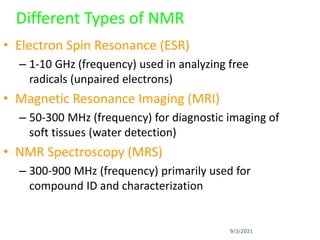
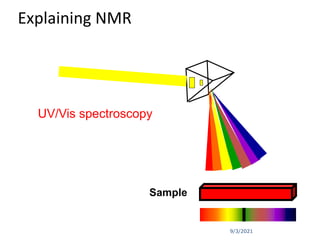
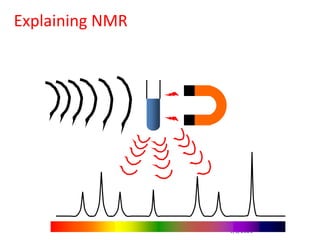
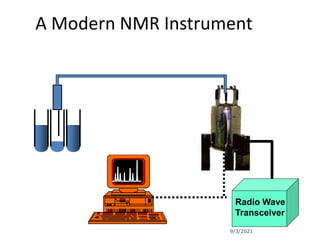
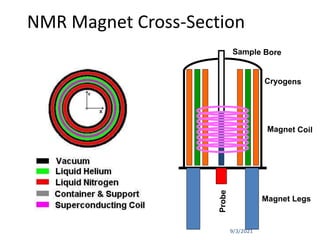
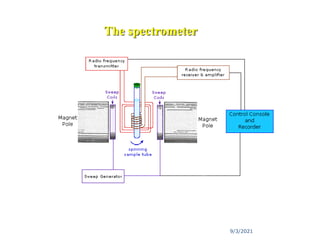
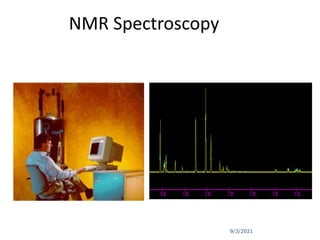
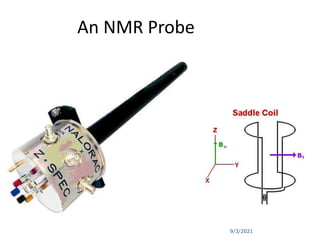
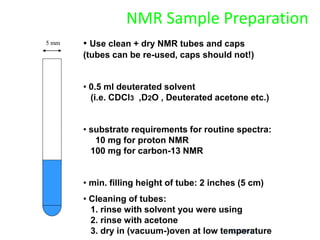
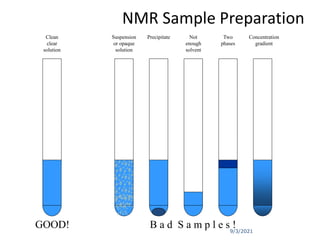
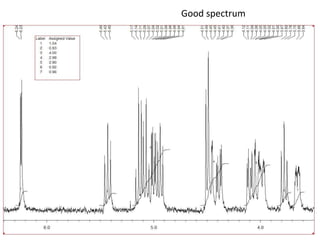
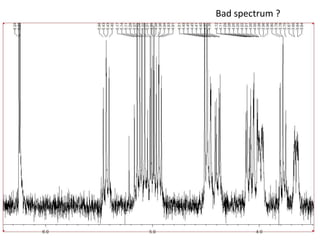
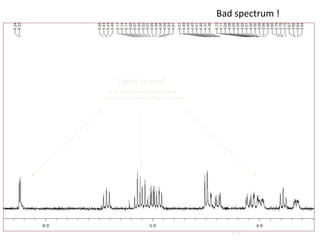
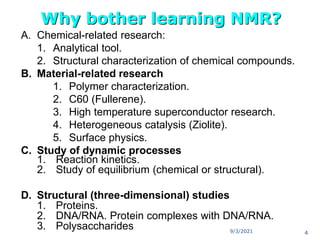
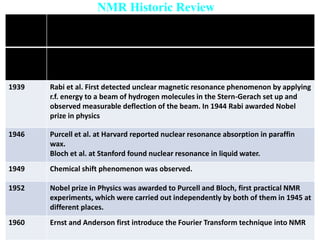
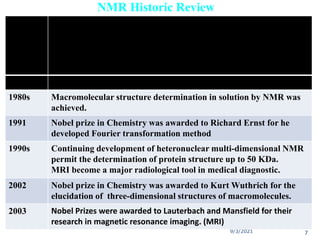
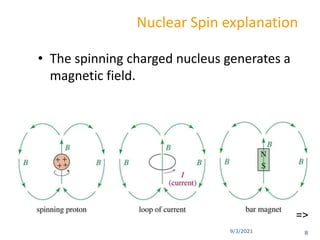
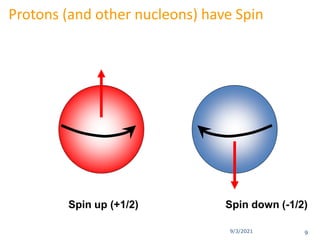
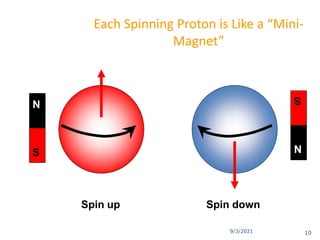
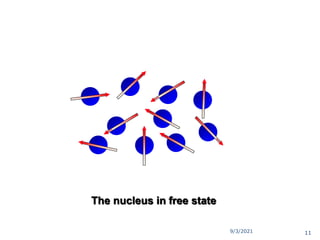
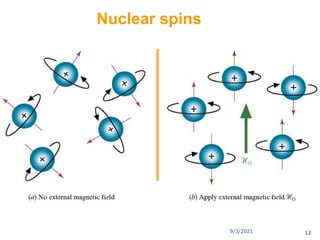
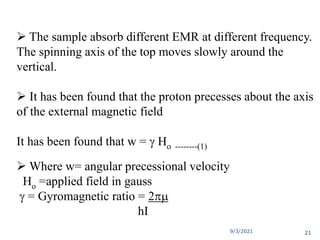
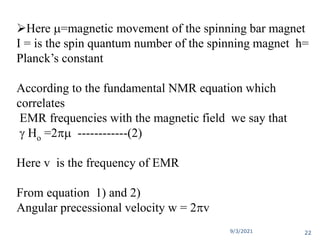
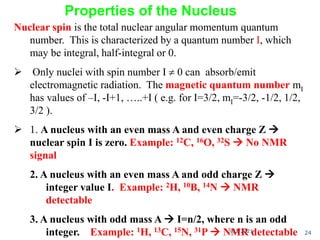
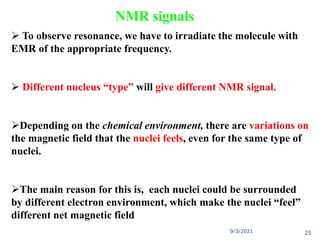
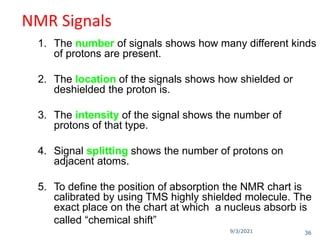
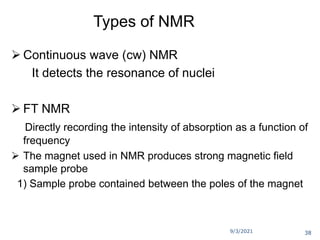
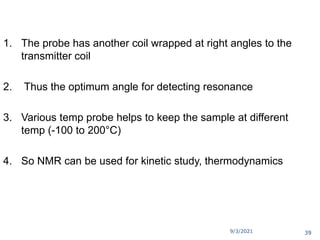
The document provides an extensive overview of nuclear magnetic resonance (NMR) spectroscopy, detailing its applications in chemical and material research, biomedical studies, and drug design. It reviews the historical development of NMR, including key figures and milestones, as well as the principles of nuclear spin and resonance, factors affecting sensitivity, and chemical shifts. Additionally, it discusses the types of NMR techniques and the significance of various signals in interpreting molecular structures.







![9/3/2021 40
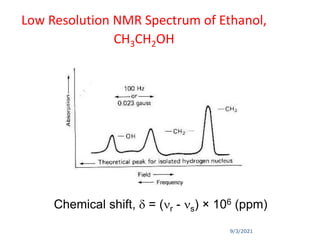
The chemical shift (δ) is defined as the difference between
the resonance position of a sample nucleus and that of a
standard TMS.
Chemical shift (δ) =[Δν (Hz)/Applied resonance frequency ×
106 Hz)] × 106 ppm
where, Δν = Difference in frequency (Hz) between the
observed signal and that of the standard.
Convention for δ : TMS assigned (δ = 0), values for other
protons are measured positively downfield.
In other words, increasing δ corresponds to increasing de-
shielding of the nucleus.](https://image.slidesharecdn.com/1hnuclearmagneticresonance-210903155959/85/1-H-Nuclear-Magnetic-Resonance-40-320.jpg)