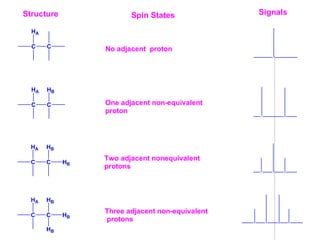
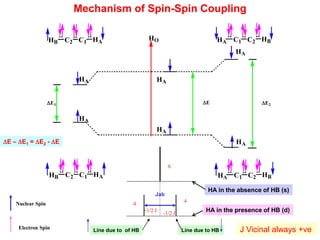
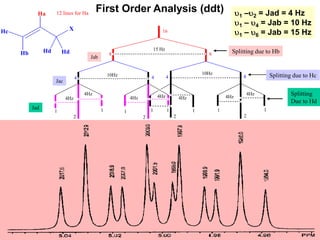
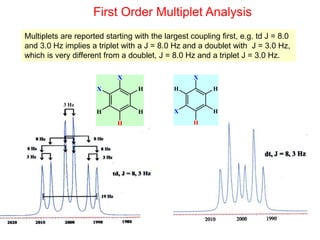
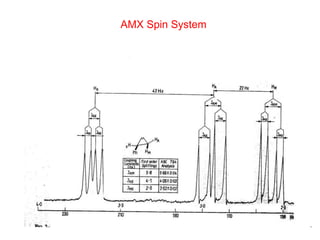
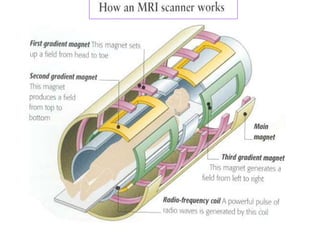
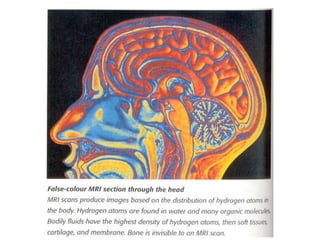
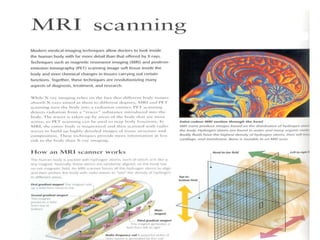
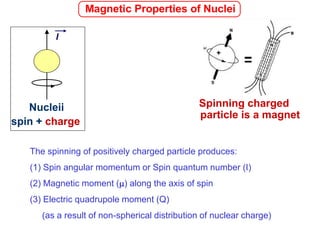
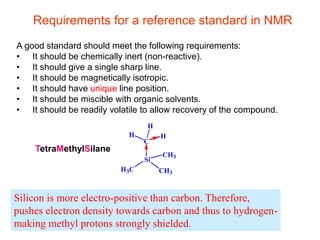
1. The document discusses the basic principles of Nuclear Magnetic Resonance (NMR) spectroscopy. It explains how certain nuclei can have spin and magnetic moments, and how these nuclei absorb electromagnetic radiation in the radiofrequency region when in a strong magnetic field.
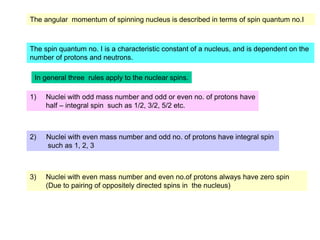
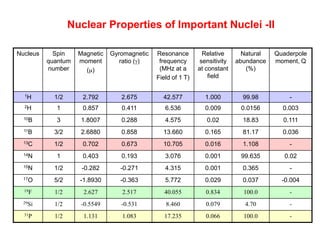
2. The key parameters that determine whether a nucleus can be observed by NMR are discussed, including spin quantum number, magnetic moment, natural abundance, and quadrupole moment. Common nuclei observed by NMR like 1H, 13C, 19F, and 31P are described.
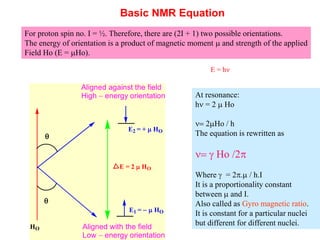
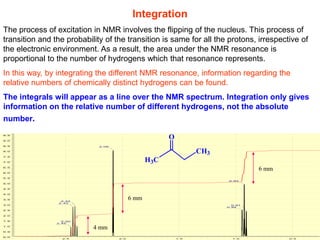
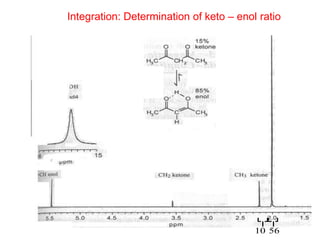
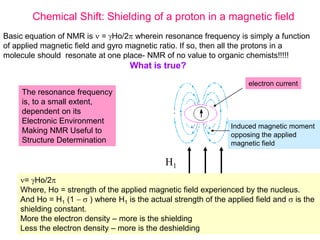
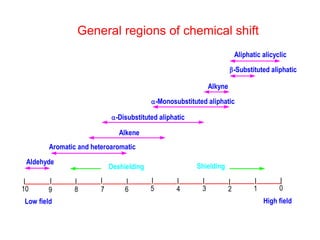
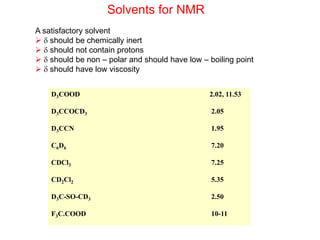
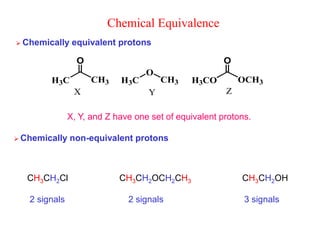
3. The basic NMR equation is presented, showing the relationship between resonance frequency, magnetic field strength, and the gyromagnetic ratio. Important NMR parameters like chemical shift, integration, and





















![Types of Coupling
Three types of coupling in NMR
Geminal Coupling [1,3 coupling]:
Coupling between nuclei on the
same carbon (Two bond coupling)
Vicinal Coupling [1,4 coupling]:
Coupling between nuclei on the adjacent
carbon atom (Three bond coupling)
Long range Coupling [1,5; 1,6- coupling]
Coupling between nuclei separated by
more than three carbon atoms
(Coupling between nuclei separated by
more than three bonds)
C C
H
H
1
2
3
4
Coupling constant ‘J’ always expressed in Hz
It is independent of applied magnetic field/Operating frequency
C C
H
C
1
2
4
H
3
5
C
H
H
2
1
3](https://image.slidesharecdn.com/nmr-210608124006/85/Nmr-35-320.jpg)