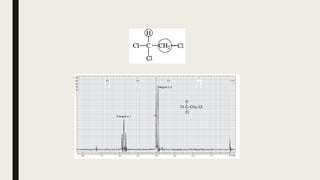
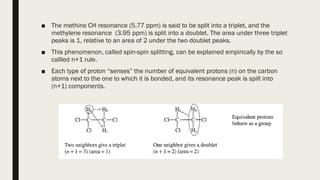
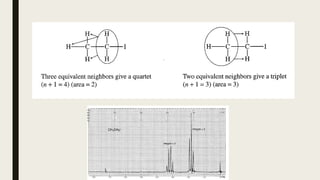
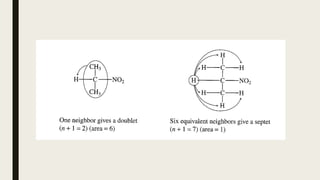
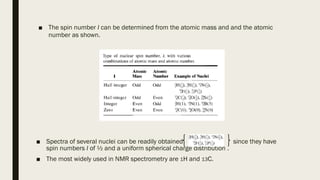
The document provides information about nuclear magnetic resonance (NMR) spectroscopy. It discusses:
1) A brief history of NMR spectroscopy from its discovery in 1945 to its application in organic chemistry structure determination and receipt of Nobel Prizes.
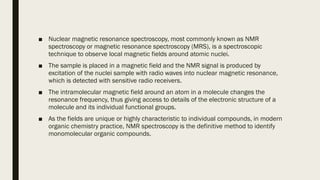
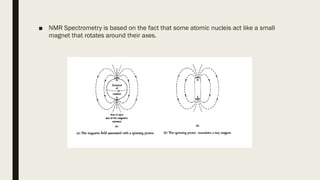
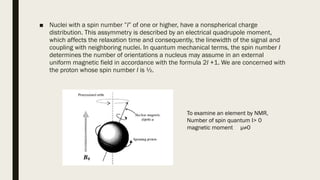
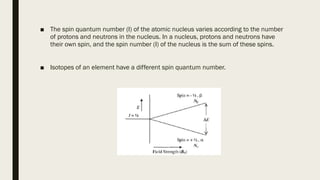
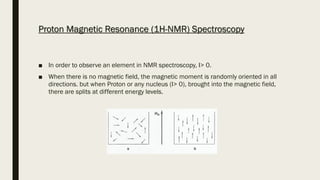
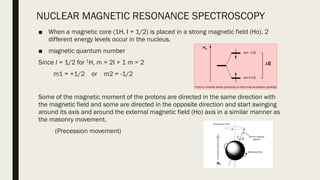
2) How NMR spectroscopy works by placing a sample in a strong magnetic field and detecting radio signals produced by excitation of atomic nuclei.
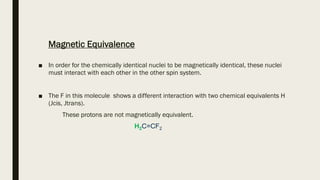
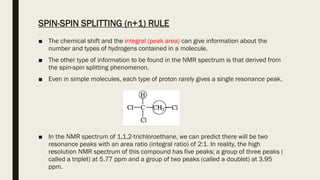
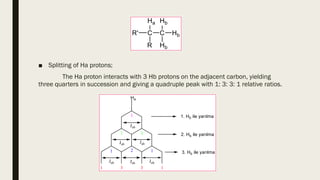
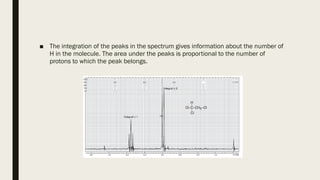
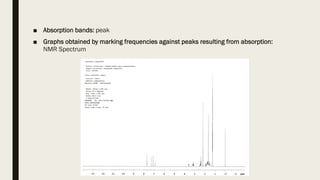
3) How NMR spectra provide information about molecular structure by revealing the number of magnetically distinct atoms and details about atomic environments.
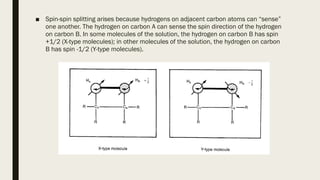
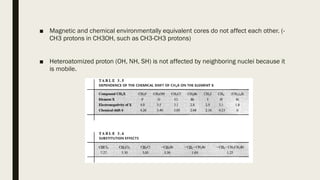
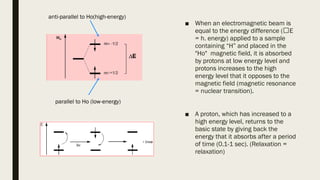
4) Factors that affect chemical shifts observed in NMR spectra, including electronegativity, hybridization, and magnetic anisotropy effects.















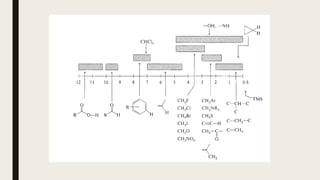




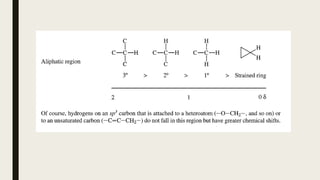
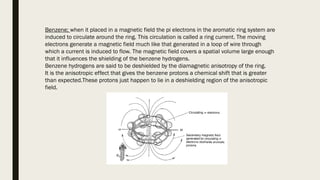
![Factors Affecting Chemical Shift
■ Local diamagnetic effects generated by electrons around the protons
■ Effect of magnetic fields generated by neighboring atomic and atomic groups
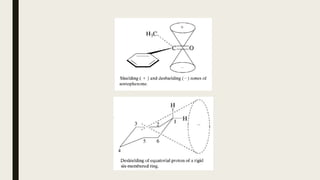
■ Magnetic anisotropy
■ Carbon hybridization [sp3 (-CH3) <sp2 (-CH2) <sp2 (Ar-H)]
■ Electronegativity of substituents
■ O (3.5)> N (3.0)> S (2.5)
■ F (4.0)> Cl (3.0)> Br (2.8)> I (2.5)
■ The sum of electronic effects
■ (Paramagnetic shift increases in proportion to the number of H substituted with
halogens having higher electronegativity.)](https://image.slidesharecdn.com/7pr-nmrspectroscopy-230207075636-b1ef6cce/85/7pr-NMR-spectroscopy-pdf-33-320.jpg)