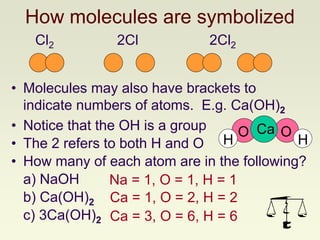
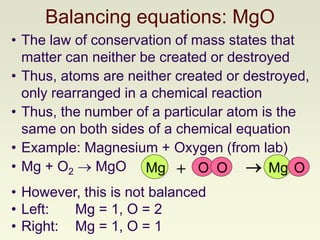
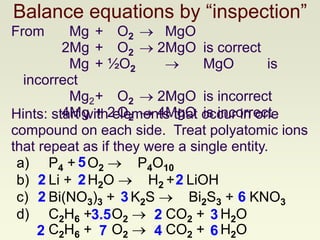
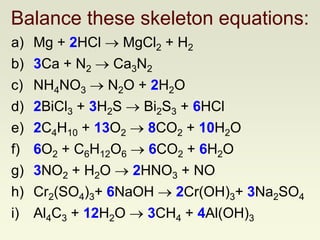
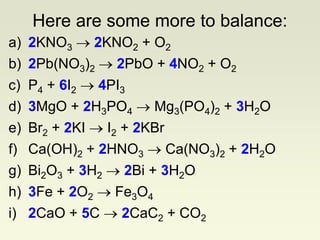
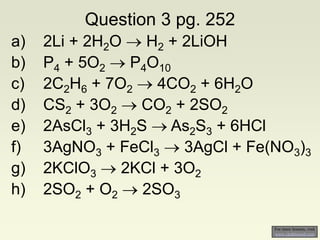
The document explains how to symbolize molecules, indicating the number of atoms in compounds and providing examples of molecular formulas. It discusses the law of conservation of mass, emphasizing that atoms are rearranged in chemical reactions and must be balanced on both sides of a chemical equation. The document includes various exercises for balancing chemical equations using a method of inspection.