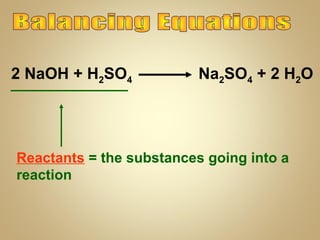
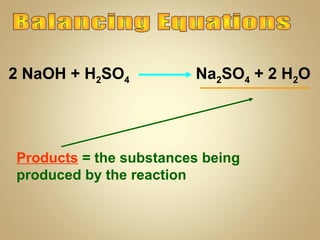
This document contains instructions and information for a chemistry class. It lists assignments that are due, including balancing equations and acids and bases work. It also provides definitions for vocabulary words and steps for balancing chemical equations. Balancing equations involves making an inventory of elements and adding coefficients to make the elements equal on both sides of the equation. The document ends by assigning homework to balance 11 equations by Monday and informs students that final exams will start on Tuesday.