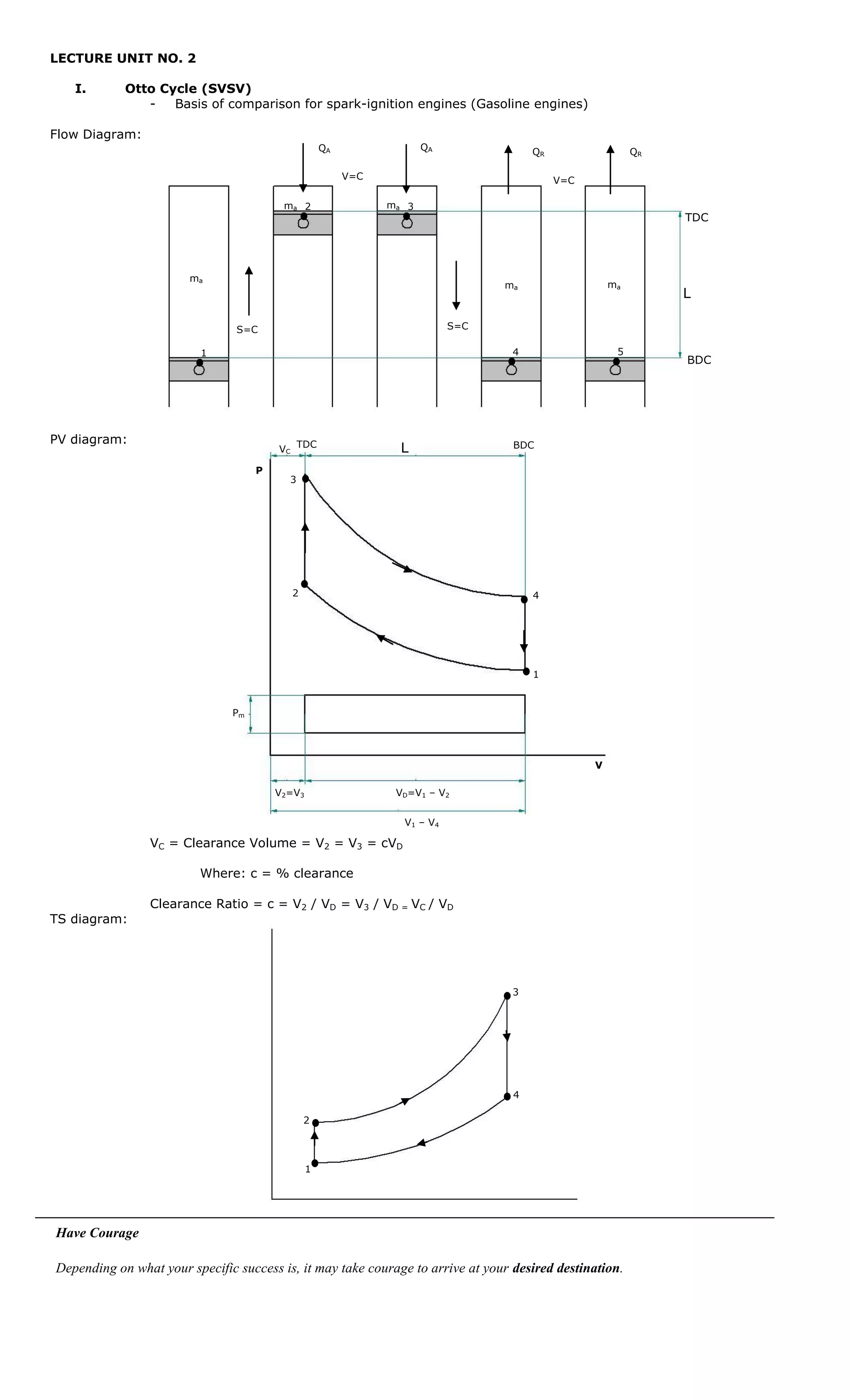
This document provides information about the Otto cycle, which is the ideal thermodynamic cycle that models the processes in a spark-ignition internal combustion engine.
It includes:
- A flow diagram and PV diagram of the Otto cycle processes
- Equations for calculating temperature, pressure, heat transfer, work, efficiency, and mean effective pressure at each state point
- Two example problems applying the Otto cycle equations
- Key parameters like compression ratio, heat added, expansion ratio, and state variables
The goal is to analyze the thermodynamics of the ideal Otto cycle as a basis for comparing spark-ignition engines. Sample calculations are provided to illustrate applying the cycle equations.