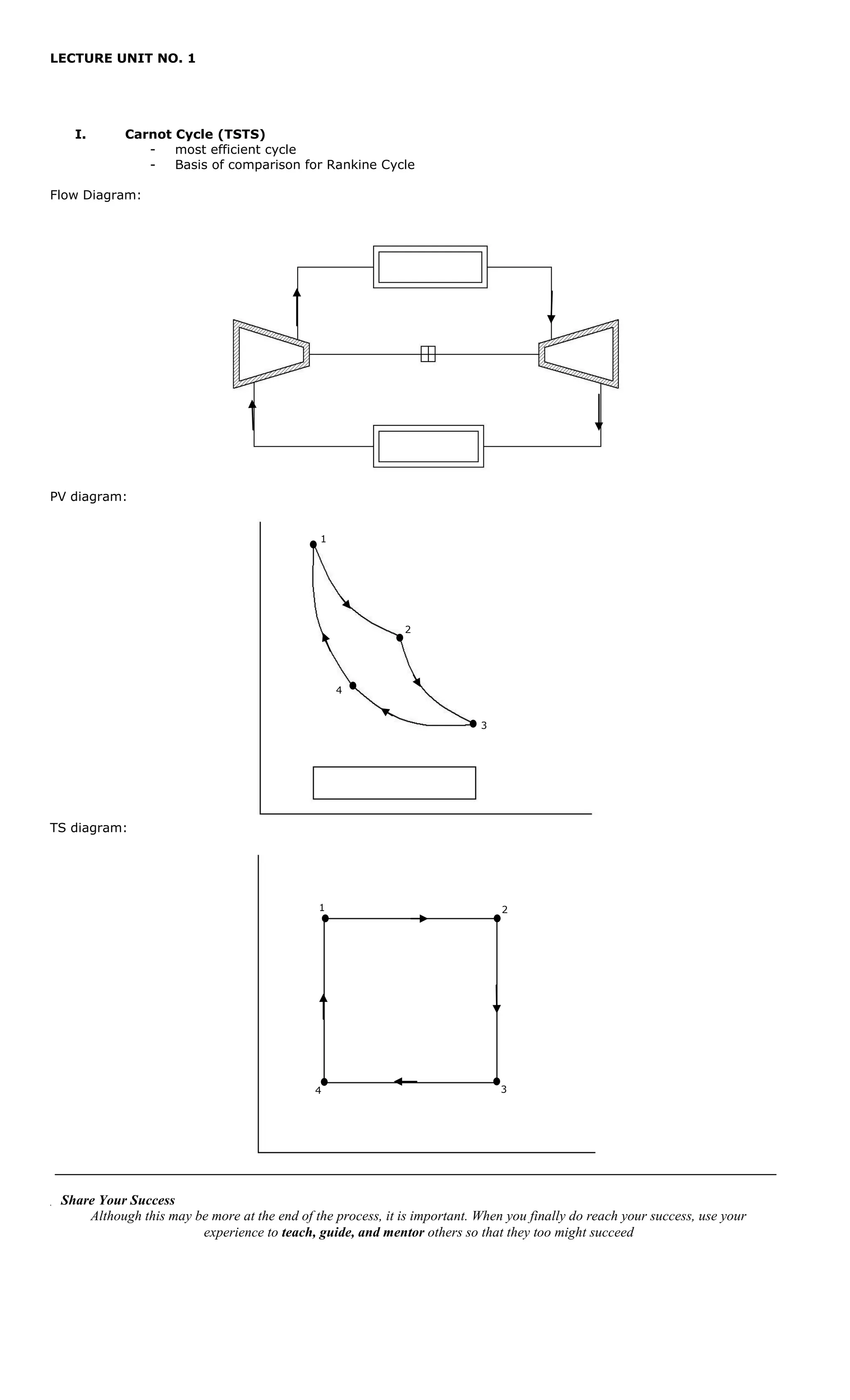
This document provides information on the Carnot cycle, including its key processes and equations. It begins with an overview of the Carnot cycle as the most efficient thermodynamic cycle, used as the basis of comparison for other cycles like the Rankine cycle. Diagrams of the pressure-volume and temperature-entropy processes are included. Key definitions and equations relating to the Carnot cycle processes are then provided, such as expansion/compression ratios and equations for heat transfer, work, and thermal efficiency. Sample problems are given at the end to demonstrate use of the equations.