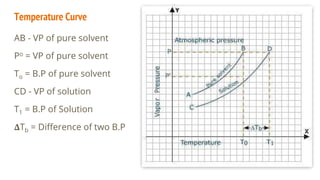
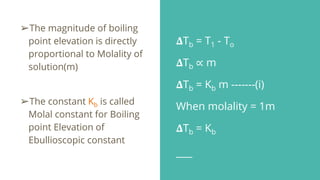
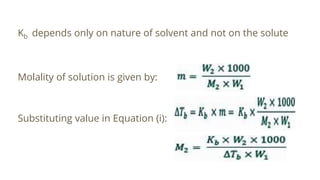
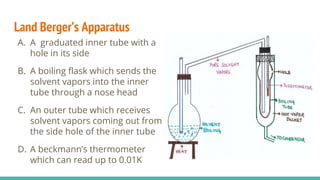
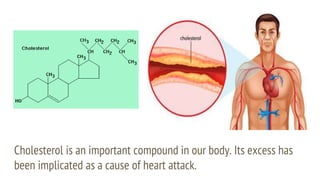
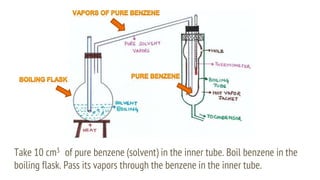
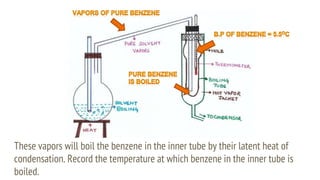
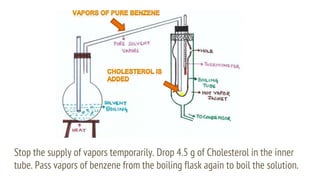
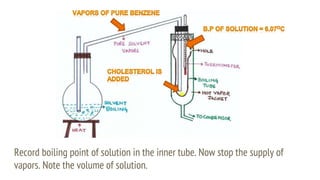
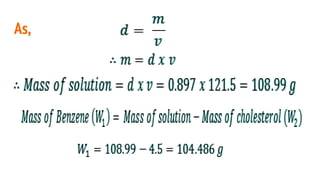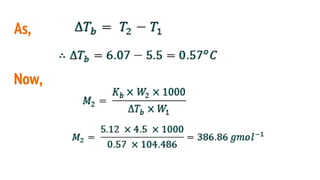This document discusses boiling point elevation, stating that the boiling point of water increases by 0.52°C for each mole of non-volatile, non-electrolyte solute added per kilogram of solvent. It describes the relationship between boiling point elevation and molality, and details a method to determine the boiling point using a Landsberger apparatus with experimental procedures involving benzene and cholesterol. The document concludes with providing specific values and formulas related to the boiling points and densities of the solutions tested.