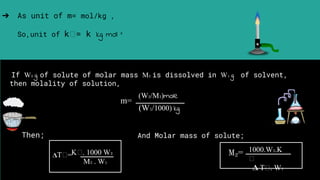
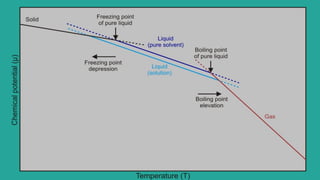
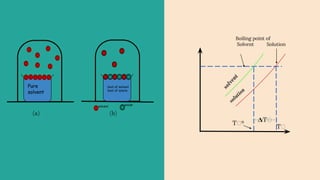
Abhay Pandey completed a chemistry project on the elevation of boiling point. They thank various people who provided guidance and support including their chemistry teacher Mrs., school principal Mr., parents, friends, and classmates. The document discusses that the boiling point of a solution is higher than that of the pure solvent alone due to the lower vapor pressure of the solution. It provides an example calculation of how to determine the boiling point of a solution using the molality of the solute and boiling point elevation constant.





![➔ For dilute Solution 𝚫Tₕ∝m
𝚫Tₕ=kₕ
mₕ
➔ If T ⁰ is Boiling point of pure solvent and T
is the boiling point of solution,then elevation
in Boiling Point.
𝚫Tₕ=Tₕ-Tₕ⁰
[Here m is molality or molar concentration of solute in a solution]
[ kₕ: Boiling Point elevation constant / Molal elevation Constant ]](https://image.slidesharecdn.com/chemistryppt-210212134306/85/elevation-in-boiling-point-8-320.jpg)